Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7350
Revised: May 28, 2006
Accepted: July 22, 2006
Published online: December 7, 2006
AIM: To explore the effects of ketamine on hemo-dynamics, plasma proinflammatory cytokine (TNF-α and IL-6) levels and nuclear factor kappa B (NF-κB) activation during polymicrobial sepsis.
METHODS: Male Sprague-Dawlay rats were subjected to cecal ligation and puncture (CLP) or sham operation. The rats were randomly assigned into four equal groups: sham CLP group, CLP group, ketamine (KT)Igroup and KTIIgroup. Thirty minutes before CLP, ketamine (5 mg/kg per hour and 10 mg/kg per hour, respectively) was infused continuously through the left femoral vein cannula in KTIgroup or KTIIgroup. Sham CLP group and CLP group received 0.9% saline only (5 mL/kg per hour). The right femoral artery was cannulated to monitor mean arterial pressure (MAP) and heart rates (HR),and draw blood samples. The proinflammatory cytokine (TNF-α and IL-6) levels of plasma were measured using enzyme-linked immunosorbent assays (ELISA). The hepatic NF-κB activation was determined by Western blot and HPIAS 2000 image analysis system. Twenty hours after CLP, the rats were killed by right femoral artery phlebotomization.
RESULTS: CLP produced progressive hypotension, and a first increase followed by a decrease in HR. The hypotension was prevented, and the HR was slightly steady in ketamine treated rats. TNF-α levels of plasma reached a peak value at 2 h after CLP. Ketamine (KTIgroup or KTIIgroup) caused a significant decrease compared with CLP group at 2, 5 and 9 h time points after CLP (14.3 ± 1.9 vs 4.3 ± 0.9, 9.7 ± 1.4 vs 4.3 ±0.9; 9.3 ± 1.5 vs 4.3 ± 0.9, 8.7 ± 1.4 vs 4.3 ± 0.9; 6.0 ± 1.5 vs 5.0 ± 1.7, 5.3 ± 0.8 vs 5.0 ± 1.7; P < 0.01, respectively). The IL-6 levels of plasma firstly ascended and then descended in CLP group, and reached a peak value at 9 h after CLP. Ketamine (KTIgroup or KTII group) caused a significant decrease compared with CLP group at 5, 9 or 20 h after CLP (135.0 ± 52.6 vs 60.0 ± 16.3, 112.5 ± 52.6 vs 60.0 ± 16.3; 410.0 ± 68.7 vs 62.5 ± 12.5, 250.0 ± 28.0 vs 62.5 ± 12.5; 320.0 ± 25.9 vs 52.5 ± 10.1, 215.0 ± 44.6 vs 52.5 ± 10.1; P < 0.05, respectively). The IL-6 levels of plasma in KTIIgroup were lower than those of KTIgroup at 9 h after CLP (250.0 ± 28.0 vs 410.0 ± 68.7; P < 0.05). In addition, CLP increased hepatic NF-κB expression compared with sham CLP. Ketamine suppressed NF-κB activation in a dose-dependent manner at 4 h after CLP (237.7 ± 3.5 vs 246.9 ± 3.1; P < 0.05).
CONCLUSION: Ketamine stabilizes the hemodynamics, attenuates the proinflammatory cytokine responses, and inhibits hepatic NF-κB activation. These findings suggest that ketamine has protective effects against polymicrobial sepsis in rats.
- Citation: Song XM, Li JG, Wang YL, Zhou Q, Du ZH, Jia BH, Ke JJ. Effects of ketamine on proinflammatory cytokines and nuclear factor kappaB in polymicrobial sepsis rats. World J Gastroenterol 2006; 12(45): 7350-7354
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7350.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7350
Sepsis/Septic shock is a complex pathophysiologic process characterized by profound hypotension, progressive metabolic acidosis, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), and even death. Although its pathophysiology is not well defined, that monocytes orchestrate the innate immune response to Gram-positive and Gram-negative bacteria by expressing a variety of inflammatory cytokines, especially tumor necrosis factor-α (TNF-α) is considered to play an important role in the pathogenesis of sepsis/septic shock[1,2].
It is known that nuclear factor kappa B (NF-κB) is an inducible nuclear transcription factor that plays a central role in regulating the transcription of proinflammatory cytokines[3], such as TNF-α, interleukin 6 (IL-6), adhesion molecules, the other mediators involved in SIRS, severe sepsis, septic shock and MODS.
Recently, several investigators have reported that intravenous anesthetic ketamine has inhibitory effects on lipopolysaccharide (LPS)-induced TNF-α production in endotoxin-shock rats[4,5]. Others have documented that ketamine could suppress proinflammatory cytokine production in human whole blood in vitro[6,7]. Thus we hypothesized that ketamine could inhibit proinflammatory cytokine responses and NF-κB activation after cecal ligation and puncture (CLP, a model of polymicrobial sepsis) challenge during polymicrobial sepsis in rats.
Polymicrobial sepsis was induced in male Sprague-Dawley (SD) rats (200-250 g) by CLP according to the method of Chaudry[8]. The rats were anesthetized with 20% ethyl carbamate (1 g/kg). Then the cecum was isolated and ligated with a 3-0 silk ligature just distal to the ileocecal valve, punctured twice at the opposite ends with a 9-gauge needle, and returned into the abdominal cavity. Following this, the abdominal incision was closed in two layers and the rats received normal saline solution (3 mL/100 g) subcutaneously.
Rats were randomly assigned into four equal groups: CLP group, sham operation group, KTI(ketamineI) group and KTII(ketamineII) group. In CLP group, thirty minutes before CLP rats received 0.9% saline through the left femoral vein cannula. Polymicrobial sepsis was induced by CLP operation. In sham CLP group, rats were subjected to the same surgical procedure except that the cecum was neither ligated nor punctured. In KTIand KT IIgroups, ketamine (5 mg/kg per hour and 10 mg/kg per hour, respectively) was infused continuously through the left femoral vein cannula 30 min before CLP. The right femoral artery was cannulated to monitor the mean arterial pressure (MAP), heart rates (HR) and draw blood samples in all groups.
At 2, 5, 9 and 20 h after CLP or sham CLP operation, blood was drawn via the right femoral artery (1.0 mL each). The plasma was immediately separated by centrifugation at 3000 rotation per min for 15 min at 4°C, divided into aliquots and stored at -70°C until assayed. The proinflammatory cytokine levels of plasma were quantified using enzyme-linked immunosorbent assays (ELISA)(American R & D and Bender).
Fresh liver tissue of 0.1 g was homogenized in buffer A (10 mmol/L HEPES-NaOH pH 7.9, 1 mmol/L EDTA, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L PMSF, 1 mmol/L DTT, 1 μg/mL Leupeptin), and the homogenates were incubated on ice (15 min) with gentle agitation. After lysis, nuclei were collected by centrifugation (14 000 rpm for 5 min). Nuclear proteins were extracted by incubation of nuclei on ice (30 min) in hypertonic salt buffer B (20 mmol/L HEPES-NaOH, pH 7.9, 0.2 mmol/L EDTA, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 25% glycerin 0.5 mmol/L PMSF, 0.5 mmol/L DTT, 1 μg/mL Leupeptin). Extracts were centrifuged and nuclear extract supernatant was harvested and stored at -70°C. Proteins were quantified by the Lowry-Kalckar assays.
The nuclear protein extracts of the liver tissue (20 μg) were boiled in Laemmli sample buffer and subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Before transfer, gels were equilibrated for 15 min in cathode buffer. Proteins were transferred at 0.8 mA/cm for 2 h onto NC membranes, preequilibrated in methanol using a semidry blotting apparatus. Equal loading and transfer were monitored by immersing ponceau S staining of the membranes. Nonspecific binding sites were blocked by immersing the membrane into blocking solution overnight at 4°C. Membranes were washed three times (10 min each) in TBST and incubated in 1:200 dilution of rabbit-antirat NF-κB p65 polyclonal antibody in blocking solution for 1 h at room temperature, followed by extensive washing three times (10 min each) with TBST. Bound antibody was decorated with 1:1000 goat-antirabbit IgG diluted in blocking solution for 30 min at room temperature, after washing four times (10 min each). Immunocomplexes were detected using DAB staining, Western blotting reagents and determined using HPIAS 2000 image analysis system.
Data were presented as mean ± SD. Analysis of variance (ANOVA) was used to evaluate whether values at the same time point were different for the control and ketamine groups and two-way analysis of variance for repeated measurements with multiple comparisons. A significant difference was presumed at a P value < 0.05.
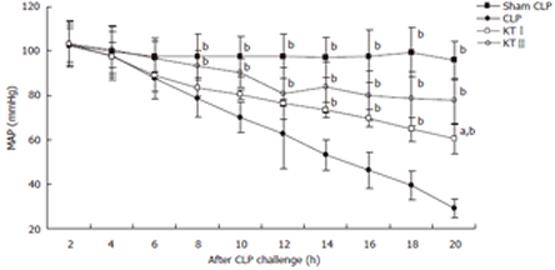
No significant differences were noted in baseline MAP among all groups. CLP challenge decreased MAP in CLP group, KTIgroup and KTIIgroup but not in sham CLP group. At 20 h time point after CLP challenge, MAP decreased by 6.8%, 71.7%, 41.5% and 24.7% in sham CLP, CLP, KTI, and KTIIgroups, respectively. There were significant differences between CLP group and KTIgroup at 14, 16, 18 and 20 h time points (P < 0.05, respectively). At the same time significant differences in MAP were found between the CLP group and KTIIgroup at 8, 10, 12, 14, 16, 18 and 20 h time points after CLP challenge during polymicrobial sepsis (P < 0.05, respectively) (Figure 1) .
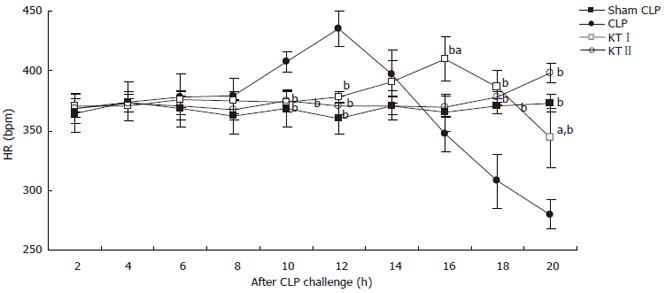
There were no significant differences in baseline HR among all groups. First increased followed by a decrease in HR after CLP challenge, HR was slightly steady in ketamine treated rats. There were significant differences in HR at 10, 12, 16, 18, and 20 h time points between CLP group and KTIgroup (P < 0.05, respectively). Significant differences in HR were found between CLP group and KTIIgroup at 10, 12, 18 and 20 h time points after CLP challenge during polymicrobial sepsis (P < 0.05, respectively) (Figure 2).
TNF-α levels of plasma were significantly increased after CLP challenge. In CLP group, TNF-α levels reached a peak level at 2 h after CLP operation (27.3 ± 3.1 pg/mL). Ketamine (KTIgroup or KTIIgroup) caused a significant decrease compared with CLP group at 2, 5 and 9 h after CLP challenge (P < 0.05, respectively). TNF-α levels in KT IIgroup were lower than those of KTI group at 2 h after CLP challenge (P < 0.05) (Table 1).
IL-6 levels of plasma were firstly increased followed by a decrease during polymicrobial sepsis in CLP group, and reached a peak level at 9 h after CLP (883 ± 127 pg/mL). Ketamine (KTIgroup or KTIIgroup) caused a significant decrease compared with CLP group at 5, 9 and 20 h after CLP challenge (P < 0.05, respectively). IL-6 levels in KTIIgroup were lower compared with those of KTIgroup at 9 h after CLP challenge (P < 0.05, respectively) (Table 2).
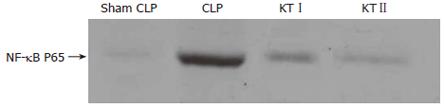
Western blot analysis demonstrated that hepatic NF-κB activity was obviously increased in CLP group (266 ± 7, absorbency) compared with sham CLP group (P < 0.05) at 4 h after CLP. NF-κB activation in KTIgroup or KTIIgroup was significantly decreased after ketamine treatment (P < 0.05, respectively). By contrast, NF-κB activation in KTIIgroup was lower than those in KTIgroup (P < 0.05) (Table 3 and Figure 3).
In our study, CLP produced progressive hypotension and obvious increase in plasma cytokine concentrations. Ketamine administration inhibited development of hypotension and increase of cytokine concentrations, which is in accordance with the literature[4-7]. It indicates that a subanesthetic dose of ketamine could inhibit endotoxin-induced expression of TNF-α and other proinflammatory mediators. We found that CLP provoked a transient elevation of TNF-α and IL-6 concentration in the plasma and enhanced the hepatic NF-κB activation in polymicrobial sepsis rats. In contrast, in the presence of ketamine, polymicrobial sepsis had inhibitory effects on the production of cytokines. Moreover, ketamine decreased the hepatic NF-κB activation during polymicrobial sepsis. This inhibitory effect of ketamine was the most important finding of the present study.
Since TNF-α is considered to play a key role in the pathogenesis of sepsis/septic shock, we detected TNF-α concentrations of plasma in rats. In addition, we also examined IL-6 concentrations. In 1994, Takenaka discovered that intravenous anesthetic ketamine had inhibitory effects on LPS-induced TNF-α production in endotoxin-induced shock in mice in vivo[3]. Following that more studies discovered that ketamine suppressed TNF-α mRNA expression and proinflammatory cytokine IL-6 and IL-8 production in human whole blood in vitro[10]. Our study demonstrated that ketamine had the same inhibitory effects on TNF-α and IL-6 expression during polymicrobial sepsis.
NF-κB is an inducible nuclear transcription factor that plays a central role in regulating the transcription of proinflammatory cytokines[3], including TNF-α, IL-6. However, the present study did not determine the cellular source of the cytokines. Numerous studies have implicated macrophages and other immunocompetent cells as important sources of proinflammatory cytokines in various models of shock and sepsis. Some investigators suggested that the hepatic Kupffer cells are a major source of proinflammatory cytokine release during acute phase of sepsis[11]. We attempted to identify the hepatic NF-κB activation during polymicrobial sepsis.
Until now, the effects of ketamine on NF-κB have been reported limitedly. In 2000, Sakai discovered ketamine inhibited endotoxin-induced NF-κB expression in brain cells in vivo and in vitro, in a dose-dependent manner[12]. In 2004, Sun discovered that ketamine suppressed endotoxin-induced NF-κB activation and TNF-α expression in the intestine, lung and liver[13]. In 2005, Suliburk found that ketamine attenuated liver injury attributed to endotoxemia: a role of cyclooxygenase-2[14]. In addition, Yang found that large dose ketamine inhibited LPS-induced acute lung injury in rats[15]. Our study indicated ketamine suppressed the hepatic NF-κB activation after CLP challenge during polymicrobial sepsis.
The doses of ketamine used in this study were 5 mg/kg per hour and 10 mg/kg per hour. Compared with the subanesthetic dose or the anesthetic dose, these doses of ketamine were relatively higher. It might be species-dependent. The two doses both inhibited proinflammatory cytokine responses and hepatic NF-κB activation, especially higher dose ketamine treatment group. It indicates that the effects of ketamine might be dose-dependent.
In summary, the current studies show that administra-tion of ketamine has protective effects against polymi-crobial sepsis in rats. Ketamine probably inhibits NF-κB activation, and attenuates the proinflammatory cytokine response. However, the precise mechanisms underlying the inhibitory effects are still unknown. Further investigations are needed, such as on toll-like receptors[16,17].
S- Editor Wang J L- Editor Zhu LH E- Editor Bai SH
| 1. | Matot I, Sprung CL. Definition of sepsis. Intensive Care Med. 2001;27 Suppl 1:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Glauser MP. Pathophysiologic basis of sepsis: considerations for future strategies of intervention. Crit Care Med. 2000;28:S4-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Abraham E. NF-kappaB activation. Crit Care Med. 2000;28:N100-N104. [PubMed] |
| 4. | Takenaka I, Ogata M, Koga K, Matsumoto T, Shigematsu A. Ketamine suppresses endotoxin-induced tumor necrosis factor alpha production in mice. Anesthesiology. 1994;80:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Taniguchi T, Shibata K, Yamamoto K. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology. 2001;95:928-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Kawasaki T, Ogata M, Kawasaki C, Ogata J, Inoue Y, Shigematsu A. Ketamine suppresses proinflammatory cytokine production in human whole blood in vitro. Anesth Analg. 1999;89:665-669. [PubMed] |
| 7. | Kawasaki C, Kawasaki T, Ogata M, Nandate K, Shigematsu A. Ketamine isomers suppress superantigen-induced proinflammatory cytokine production in human whole blood. Can J Anaesth. 2001;48:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1087] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1912] [Cited by in RCA: 2047] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 10. | Hoff G, Bauer I, Larsen B, Bauer M. Modulation of endotoxin-stimulated TNF-alpha gene expression by ketamine and propofol in cultured human whole blood. Anaesthesist. 2001;50:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Koo DJ, Zhou M, Jackman D, Cioffi WG, Bland KI, Chaudry IH, Wang P. Is gut the major source of proinflammatory cytokine release during polymicrobial sepsis. Biochim Biophys Acta. 1999;1454:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sakai T, Ichiyama T, Whitten CW, Giesecke AH, Lipton JM. Ketamine suppresses endotoxin-induced NF-kappaB expression. Can J Anaesth. 2000;47:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Sun J, Li F, Chen J, Xu J. Effect of ketamine on NF-kappa B activity and TNF-alpha production in endotoxin-treated rats. Ann Clin Lab Sci. 2004;34:181-186. [PubMed] |
| 14. | Suliburk JW, Helmer KS, Gonzalez EA, Robinson EK, Mercer DW. Ketamine attenuates liver injury attributed to endotoxemia: role of cyclooxygenase-2. Surgery. 2005;138:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yang J, Li W, Duan M, Zhou Z, Lin N, Wang Z, Sun J, Xu J. Large dose ketamine inhibits lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2005;54:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Brightbill HD, Modlin RL. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology. 2000;101:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2272] [Cited by in RCA: 2261] [Article Influence: 87.0] [Reference Citation Analysis (0)] |