Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7341
Revised: September 28, 2006
Accepted: November 6, 2006
Published online: December 7, 2006
AIM: To analyze the results of ultrasound guided percu-taneous needle aspiration (PNA) and percutaneous catheter drainage (PCD) in the treatment of splenic abscess.
METHODS: Thirty-six patients (14 females and 22 males, with an average age of 54.1 ± 14.1 years) with splenic abscess were treated with ultrasound guided PNA and/or PCD. Patients with splenic abscess < 50 mm in diameter were initially treated by PNA and those with abscess ≥ 50 mm and bilocular abscesses were initially treated by an 8-French catheter drainage. The clinical characteristics, underlying diseases, organism spectra, therapeutic methods, and mortality rates were analyzed.
RESULTS: Twenty-seven patients had unilocular and 9 bilocular abscess. PNA was performed in 19 patients (52.8%), and 8 of them (42.1%) required PCD because of recurrence of abscess. In 17 patients (47.2%), PCD was performed initially. PCD was performed twice in six patients and three times in two. PNA was definitive treatment for 10 and PCD for 21 patients. One patient with PCD was referred for splenectomy, with successful outcome. In all 4 deceased patients, malignancy was the underlying condition. Twenty-one patients (58.3%) underwent 33 surgical interventions on abdomen before treatment. Cultures were positive in 30 patients (83.3%). Gram-negative bacillus predominated (46.7%). There were no complications related to the procedure.
CONCLUSION: Percutaneous treatmnet of splenic abscess is an effective alternative to surgery, allowing preservation of the spleen. This treatment is especially indicative for the patients in critical condition postoperatively. We recommend PNA as primary treatment for splenic abscesses < 50 mm, and PCD for those ≥ 50 mm in diameter and for bilocular abscesses.
- Citation: Zerem E, Bergsland J. Ultrasound guided percutaneous treatment for splenic abscesses: The significance in treatment of critically ill patients. World J Gastroenterol 2006; 12(45): 7341-7345
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7341
Splenic abscess is an uncommon entity with a reported frequency in autopsy series between 0.14% and 0.7%, and with high mortality rates because of delayed detection and treatment[1-3]. It often presents with either vague or nonspecific signs, thus making clinical diagnosis difficult. Current imaging modalities allow early diagnosis. Furthermore, splenic abscess often occurs in the patients with underlying diseases[1,4-6].
Many surgeons report that splenectomy is the best way for treatment of splenic abscess[2,4,6,7]. Although antibiotics and splenectomy are traditionally considered the treatment of choice, there is wide acceptance of important immunologic support of the spleen[8-10]. Recently, spleen-preserving management using medical treatment and percutaneous imaging-guided drainage were proved to be efficient methods for the treatment of splenic abscess[8-13]. Our study was undertaken to determine the current role of percutaneous ultrasound guided treatment of splenic abscesses as an alternative to surgery, with intention to analyze the role of needle aspiration and continous catheter drainage especially in patients in critical condition postoperatively.
From January 1, 1999 to December 31, 2004, all consecutive patients who were admitted to our hospital and subjected to percutaneous treatment of splenic abscess, were included in this study. All patients gave written informed consent. This study was approved by the local ethics committee.
The diagnosis of splenic abscess was made on the basis of clinical and imaging findings with ultrasound or computed tomography. Splenic abscess was defined as an intrasplenic pus collection. Case definition required patients to have one or more defects on spleen ultrasound examination or computed tomography along with the identification of pus. The demographic, clinical, and laboratory characteristics, underlying diseases, organism spectra, abscess number and size, and mortality rates were analyzed.
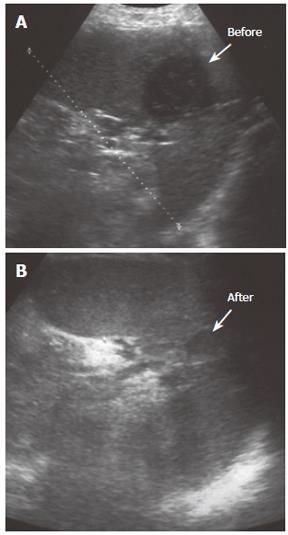
The size of abscess was recorded as the largest diameter in either solitary or bilocular abscess. The decision to select the method for percutaneous treatment between percutaneous needle aspiration (PNA) or percutaneous catheter drainage (PCD) was made on the basis of the longest diameter of abscess and number of abscesses. The patients with splenic abscess shorter than 50 mm in maximal diameter were initially treated with PNA. The reason for this is that the end of an 8-French pigtail catheter is a ring with 25 mm diameter and its insertion in a cavity shorter than its double length (50 mm in its longest diameter) would be difficult. In the case of the recurrence of abscess collection, an 8-French catheter for continous drainage was introduced. Patients with splenic abscess equal or greater than 50 mm in diameter were initially treated with PCD. All bilocular splenic abscess were treated with catheter regardless of the maximal diameter of the abscess. All surviving patients were followed up clinically for six months after percutaneous treatment. Patients’ response to treatment in terms of clinical symptoms and laboratory test results were monitored. The criteria for successful treatment were if the infection had subsided clinically and if there had been sonographic evidence of abscess resolution (Figure 1). Patients were followed up in the out-patient clinic biweekly after they discharged from the hospital. Clinical examination, white blood cell count and neutrophillia, CRP and abdominal ultrasonography were performed at each follow-up visit.
Antibiotics policy: At present, all patients were treated with intravenous ampicillin 500 mg qid, cefuroxime 750 mg tid, and metronidazole 500 mg tid. The antibiotics were adjusted according to the results of the culture and sensitivity test of pus aspirated at the time of the drainage procedure. Antibiotics adjustment was done immediately when the sensitivity test was available. Patients with negative culture results were continuously treated with combination of ampicillin, cefuroxime, and metronidasole. Intravenous antibiotics were continued for 10 d. If antibiotic therapy was changed according to the results of sensitivity test, new antibiotics were administered for 10 d. Patients were discharged earlier with a percutaneous intravenous catheter inserted for a completion of therapy if fever had subsided for at least 48 h. The patients were then put on appropriate oral antibiotics for a total treatment period of 6 wk.
Intervention: All percutaneous interventions were performed under ultrasound guidance with a General Electric Logiq 400 machine and 3.5 MHz curvilinear transducer (General Electric, Chicago, USA). A free-hand technique using an 18 Gauge disposable trocar needle (Boston Scientific, Boston, USA) of varying lengths (10-16 cm) was employed for puncturing of abscesses. A sample of pus was routinely taken and sent for microbiological analyses, including microscopy, culture and antibiotics sensitivity tests.
Percutaneous drainage: The applied drainage technique was the trocar method using an 8F multisidehole pigtail catheter (Boston Scientific, Boston, USA). by this method, reduction of catheter manipulations avoids damage of splenic tissues. Careful localization of the lesion and proper selection of the entry site were required. An optimal route of access must transverse the least possible amount of splenic parenchyma and avoid bowel and pleura.
Aspiration was then performed with the catheter until no more pus could be removed. The catheter was then secured to the skin for continuous external drainage and the patient was sent back to the ward. If after 24 h there was no catheter output, a follow-up sonography was performed. If the abscess cavity was absent, the catheter was removed. If a residual cavity was present, the catheter was flushed with saline and content aspirated until such time that the aspirated content is clear. Residual abscesses were treated with catheter repositioning and aspiration or introducing the new catheter. Subsequent sonography was performed three days later and the catheter was removed if the catheter remained unproductive. Otherwise, the catheter was left in situ until it stopped producing any content. Sonography was repeated every 3 d until the cavity either disappeared or showed significant reduction, along with clinical recovery.
Needle aspiration: Complete evacuation of pus from each cavity was attempted with 18G disposable trocar needle (Boston Scientific). The needle tip was inserted into the abscess for a complete pus removal. Sonography was performed every 3 d and the size of the abscess was recorded. If there was either no clinical improvement or no reduction in size of the abscess cavity, catheter for continuous drainage was introduced.
Statistical analysis was done using statistical software Med Calc v. 8.0. Quantitative variables were compared using two-sample t test for independent samples, whereas categorical variables were analyzed by Fischer exact test. Statistical level of P < 0.05 was considered as significant for all performed tests.
Splenic abscess was diagnosed in 41 patients during the study period. Five of them, who died within 6 wk after percutaneous treatment because of underlying diseases, were excluded. Out of the remaining 36 patients with splenic abscess, 14 were females and 22 males, with a mean (± SD) age of 54.1 ± 14.1 (range 7-69) years.
Prior to hospital admission, patients were symptomatic for a mean 16.83 ± 18.20 (range 1-72) d. The symptoms were fever in 33 patients (91.7%), left upper quadrant abdominal pain in 23 patients (63.9%), diffuse abdominal pain in 7 patients (19.4%), and shortness of breath in 6 patients (16.7%). The physical examination revealed splenomegaly in 24 patients (60%), left upper quadrant abdominal tenderness in 21 patients (58.3%), and generalized abdominal tenderness in 8 patients (22.2%). Leucocytosis over 10 000/mm³ was revealed in 32 patients (88.8%), and CRP over 10 (normal up to 3.3) mg/L in 29 patients (55.6%). Among 36 patients, a potential underlying disease for splenic abscess was found in 30 (83.3%). Acute hemorrhagic necrotic pancreatitis, trauma and malignancy were the leading diseases (8 patients affected by each, covering 22.2%) (Table 1). Statistical analysis revealed that the presence of underlying disease did not correlate with the size and number of abscess, age, gender, and species of microorganism.
| Causes | n | % |
| Pancreatitis | 8 | 22 |
| Trauma | 8 | 22 |
| Stomach surgery | 8 | 22 |
| Deviscerated hydatid cyst | 2 | 6 |
| Nephrectomia | 2 | 6 |
| Liver cirrhosis | 1 | 3 |
| Spleen infarction | 1 | 3 |
| Unknown | 6 | 16 |
Frank pus was obtained from the abscesses in all 36 patients. A microbial pathogen was isolated in 30 patients (83.3%). Blood culture was positive in 11 of 30 patients (36.6%) and abscess culture in 23 of 30 patients (83.3%). All patients with both positive blood and abscess cultures had identical pathogens. The microorganisms of positive culture were predominantly gram negative (14 cases, 46.7%), with K. pneumoniae as the leading pathogen (20%). Gram positive microorganisms were isolated in 10 patients (33.3%). More than one organism was isolated in 16.7% of patients with positive culture (Table 2). All patients received appropriate antibiotic therapy. Twelve patients after pus culture and sensitivity test were obtained, had antibiotics changed, including imipenem (n = 5), claritromycine (n = 3), ciprofloksacin (n = 2), and gentamycin (n = 2). The mean hospital stay (± SD) was 20.1 ± 14 d. The shortest hospital stay was in the group with PNA (Table 3). The mean hospital stay (± SD) was significantly shorter (P < 0.001) in 19 patients of groups PNA + PNA/PCD (12.4 ± 7.7 d) than in 17 patients of PCD groups (28.7 ± 14.7 d).
| Microorganism | n | Isolated from | ||
| Pus | Blood | Pus andblood | ||
| Aerobes | ||||
| Gram positive | ||||
| Staphyloccocus aureus (SA) | 6 | 3 | 1 | 2 |
| Streptoccocus viridans (SV) | 2 | 2 | 0 | 0 |
| Enterococcus species | 2 | 2 | 0 | 0 |
| Gram negative | ||||
| Klebsiella pneumoniae (KP) | 6 | 4 | 1 | 1 |
| Pseudomonas species | 5 | 1 | 1 | 3 |
| Escherichia coli (EC) | 3 | 2 | 1 | 0 |
| Gram positive + Gram negative SA + ECSV + KP | 3 2 | 2 2 | 1 0 | 0 0 |
| Anaerobes Bacteroides fragilis | 1 | 1 | 0 | 0 |
| Sterile culture | 6 | 0 | 0 | 0 |
| Total | 36 | 19 | 5 | 6 |
| Treatmentmethod | n | Hospitalstay (d) | Number of surgeries beforepercutaneous treatment | ||
| One | Two | Three | |||
| PNA | 11 | 9.4 ± 5.3 | 2 | 2 | 0 |
| PNA/PCD | 8 | 16.6 ± 8.7 | 4 | 0 | 0 |
| PCD x 1 | 9 | 23.9 ± 17 | 5 | 1 | 1 |
| PCD x 2 | 6 | 32.5 ± 10 | 1 | 2 | 1 |
| PCD > 2 | 2 | 39.1 ± 7.1 | 0 | 1 | 1 |
| Total | 36 | 20.1 ± 14 | 12 | 6 | 3 |
The patients had either unilocular (27 cases) or bilocular (9 cases) splenic abscess. Sizes of abscess ranged from 26 to 97 mm, with a mean of 58.9 ± 8.6 mm. Size of abscess ranged from 26 to 49 mm, with a mean 40.2 ± 5.5 mm, in the PNA group and from 57 to 97 mm, with a mean 77.5 ± 11.7 mm, in the PCD group. Twenty-one patients (58.3%) underwent 33 surgical interventions on abdomen before percutaneous treatment. Six patients had two and three patients had three surgical interventions before percutaneous treatment was applied (Table 3).
Needle aspiration was performed in 19 patients (52.8%). In 10 patients, needle aspiration was a definitive and successful treatment, and one patient died 55 d after PNA and repeated surgery due to recurrence of stomach malignancy. Eight of 19 patients (42.1%) had recurrence of abscess collection and required continuous percutaneous drainage and instilled antibiotic therapy through the catheter. In 17 patients (47.2%) with abscess collection greater than 50 mm in diameter or bilocular abscess, percutaneous catheter drainage was initially performed.
Six patients with persistent abscess cavity or poor drainage underwent percutaneous catheter drainage twice and two patients for three times. PCD was a definitive and successful treatmnet for 21 patients, seven received it after PNA and PCD, five after two PCD and two after three PCD. One patient with percutaneous drainage was eventually referred for splenectomy, with a favorable outcome (Table 4). There was no statistically significant difference in the success of percutaneous treatment between unilocular and bilocular splenic abscesses (P > 0.95). As for the imaging techniques applied for splenic abscess, all patients underwent chest X-ray. Twenty-two patients (61.1%) had left lower lung infiltration (6 patients, 16.7%) or left pleural effusion (16 patients, 44.4%). The abnormalities revealed by chest X-ray disappeared after successful treatment of the splenic abscess. The mortality rate was 11.1% (Table 4). All 4 deceased patients died of predisposing factors such as malignancy rather than of splenic abscess itself.
In the past, antibiotic therapy and splenectomy were cosidered the treatments of choice for splenic abscess[2,14]. Because of the increased number of immunocompromised patients within the general population, the incidence of splenic abscesses has increased over the last decade[15]. The spleen is important for proper immunologic function, and splenectomy carries an increased morbidity rate with the danger of postsplenectomic infections. Current therapeutic strategies established spleen-preserving treatment in cases of trauma and benign lesions. Thus, percutaneous drainage of splenic abscesses is used instead of surgical treatment with good results[9-13], provided that certain conditions were present. Contrary to these opinions, some new studies recommended splenectomy as the treatment of choice for splenic abscesses and splenic pseudocysts[2,16].
Our series revealed that gram negative bacilli were the leading pathogens causing splenic abscess. K. pneumoniae was the most frequently found pathogen. Some previous studies had similar[1], but other reports revealed different results (mailnly Streptococcus and Staphylococcus species)[4,6,7].
Percutaneous treatment is the most convenient when the abscess collection is unilocular or bilocular with a discrete wall and no internal septations, and when its content is liquid enough to be drained. As for the location, better results are obtained at the periphery and at the middle or lower pole of the spleen. The number of collections is another important factor. If there are more than two collections, surgical treatment should be considered[9]. All patients in our study had unilocoluar or bilocular abscess located at the middle or lower pole of the spleen.
Percutaneous treatment is indicated especially when patients are in critical condition postoperatively or when the risks of general anesthesia, surgical drainage, or splenectomy are substantial. Some authors report that percutaneous drainage or needle aspiration should be considered as the first line of treatment, reserving splenectomy for exceptional cases only[11-13]. More than half of patients enrolled in our study had surgical intervention in abdomen or retroperitoneum (9 after malignancy) earlier and percutaneous drainage or needle aspiration was the only available treatment for those patients. Percutaneous treatment was a successful and definitive treatment in almost all of our patients. All deceased patients died of underlying deseases of malignancies. The advantages of percutaneous treatment were compared with surgery, including external drainage without significant risks of intra-abdominal spillage and avoidance of perioperative complications, less time and cost, better acceptance by the patient, and easier nursing care. Also, immunologic disfunction after splenectomy is avoided, which is a desirable outcome, especially in young patients[9,11-13].
We recommend needle aspiration, primarily in the treatment of splenic abscess with the maximal diameter less than 50 mm, since our experience with those cases showed that needle aspiration was sufficient to solve these splenic abscesses in more than 50% cases. Also, the method is much simpler and less aggressive than percutaneous catheter drainage, allowing shorter hospital stay and lower costs. If needle aspiration was insufficient, we subsequently used the drainage technique by the trocar method using pigtail an 8-French catheters. In patients with splenic abscesses longer or equal to 50 mm in maximal diameter, we initially introduced an 8-French catheter. As for the bilocular abscesses, we always introduced a single 8-French catheter to take the advantage of the hydrostatic pressure gradient created when one of the cavities was drained, thereby causing sometimes spontaneous drainage of other cavities. If persistence of clinical signs was combined with poor drainage, the correct location of the catheter should be verified. The possibility of placing an additional catheter should always be explored if the abscess continues to exist. Complications associated with percutaneous drainage of splenic abscesses include hemorrhage, pleural empyema, pneumothorax and fistula formation[9,17]. These complications are rarely encountered if the exclusion criteria were carefully followed. In our study, all splenic abscesses were successfully drained without any complications.
In conclusion, ultrasound guided percutaneous treatment of splenic abscess is a safe and effective alternative to surgery, allowing preservation of the spleen, avoiding perioperative complications, ensuring better compliance and easier nursing care. This treatment is indicated especially when patients are in critical condition postoperatively or when the risks of general anesthesia, surgical drainage, or splenectomy are substantial. We recommend needle aspiration primarily in the treatment of splenic abscesses smaller than 50 mm and continuous percutaneous drainage for those larger than 50 mm in the longest diameter as well as for the bilocular abscesses.
| 1. | Chang KC, Chuah SK, Changchien CS, Tsai TL, Lu SN, Chiu YC, Chen YS, Wang CC, Lin JW, Lee CM. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol. 2006;12:460-464. [PubMed] |
| 2. | Tung CC, Chen FC, Lo CJ. Splenic abscess: an easily overlooked disease. Am Surg. 2006;72:322-325. [PubMed] |
| 3. | Chiang IS, Lin TJ, Chiang IC, Tsai MS. Splenic abscesses: review of 29 cases. Kaohsiung J Med Sci. 2003;19:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Nelken N, Ignatius J, Skinner M, Christensen N. Changing clinical spectrum of splenic abscess. A multicenter study and review of the literature. Am J Surg. 1987;154:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 137] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Joazlina ZY, Wastie ML, Ariffin N. Computed tomography of focal splenic lesions in patients presenting with fever. Singapore Med J. 2006;47:37-41. [PubMed] |
| 6. | Green BT. Splenic abscess: report of six cases and review of the literature. Am Surg. 2001;67:80-85. [PubMed] |
| 7. | Smyrniotis V, Kehagias D, Voros D, Fotopoulos A, Lambrou A, Kostopanagiotou G, Kostopanagiotou E, Papadimitriou J. Splenic abscess. An old disease with new interest. Dig Surg. 2000;17:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Goerg C, Schwerk WB, Goerg K. Splenic lesions: sonographic patterns, follow-up, differential diagnosis. Eur J Radiol. 1991;13:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Tikkakoski T, Siniluoto T, Päivänsalo M, Taavitsainen M, Leppänen M, Dean K, Koivisto M, Suramo I. Splenic abscess. Imaging and intervention. Acta Radiol. 1992;33:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Schäberle W, Eisele R. [Percutaneous ultrasound controlled drainage of large splenic abscesses]. Chirurg. 1997;68:744-748. [PubMed] |
| 11. | Chou YH, Tiu CM, Chiou HJ, Hsu CC, Chiang JH, Yu C. Ultrasound-guided interventional procedures in splenic abscesses. Eur J Radiol. 1998;28:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Choudhury S R, Rajiv C, Pitamber S, Akshay S, Dharmendra S. Management of splenic abscess in children by percutaneous drainage. J Pediatr Surg. 2006;41:e53-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Taşar M, Uğurel MS, Kocaoğlu M, Sağlam M, Somuncu I. Computed tomography-guided percutaneous drainage of splenic abscesses. Clin Imaging. 2004;28:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Farres H, Felsher J, Banbury M, Brody F. Management of splenic abscess in a critically ill patient. Surg Laparosc Endosc Percutan Tech. 2004;14:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wu HM, Kortbeek JB. Management of splenic pseudocysts following trauma: a retrospective case series. Am J Surg. 2006;191:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Chou YH, Hsu CC, Tiu CM, Chang T. Splenic abscess: sonographic diagnosis and percutaneous drainage or aspiration. Gastrointest Radiol. 1992;17:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Ma JY E- Editor Liu WF