Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7319
Revised: April 28, 2006
Accepted: July 18, 2006
Published online: December 7, 2006
AIM: To assess pre-orthotopic liver transplantation (OLT) factors that could be evaluated pre-operatively or controlled post-operatively associated with hepatocellular carcinoma (HCC) recurrence and disease-free survival after liver transplantation (LT).
METHODS: Four hundred and twelve patients transplanted for HCC between 1988 and 1998 in 14 French centers, who survived the postoperative period were studied. Kaplan Meier estimates were calculated for 24 variables potentially associated with recurrence of HCC. Uni- and multivariate analyses were conducted to identify independent predictors of recurrence.
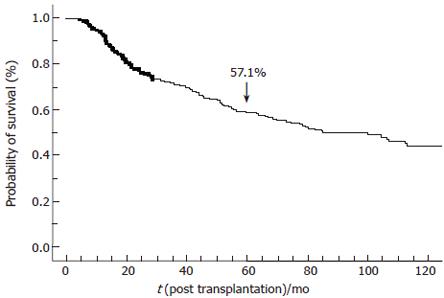
RESULTS: Overall 5-year disease-free survival was 57.1%. By univariate analysis, variables associated with disease-free survival were: presence of cirrhosis (P = 0.001), etiology of liver disease (P = 0.03), α fetoprotein level (< 200, 200 to 2000, or > 2000; P < 0.0001), γ-GT activity (N, N to 2N or > 2N; P = 0.02), the number of nodules (1, 2-3 or ≥ 4; P = 0.02), maximal diameter of the largest nodule (< 3 cm, 3 to 5 cm or > 5 cm; P < 0.0001), the sum of the diameter of the nodules (< 3 cm, 3 to 5 cm, 5 to 10 cm or >10 cm; P < 0.0001), bi-lobar location (P = 0.01), preoperative portal thrombosis (P < 0.0001), peri-operative treatment of the tumor (P = 0.002) and chemoembolization (P = 0.03), tumor differentiation (P = 0.01), initial type of calcineurin inhibitor (P = 0.003), the use of antilymphocyte antibodies (P = 0.02), rejection episodes (P = 0.003) and period of LT (P < 0.0001). By multivariate analysis, 6 variables were independently associated with HCC recurrence: maximal diameter of the largest nodule (P < 0.0001), time of LT (P < 0.0001), tumor differentiation (P < 0.0001), use of anti-lymphocyte antibody (ATG) or anti-CD3 antibody (OKT3) (P = 0.005), preoperative portal thrombosis (P = 0.06) and the number of nodules (P = 0.06).
CONCLUSION: This study identifies immunosuppression, through the use of ATG or OKT3, as a predictive factor of tumor recurrence, and confirms the prognostic value of tumor differentiation.
- Citation: Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Compagnon P, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Dharancy S, Chazouillères O, Cherqui D, Duvoux C. Role of immunosuppression and tumor differentiation in predicting recurrence after liver transplantation for hepatocellular carcinoma: A multicenter study of 412 patients. World J Gastroenterol 2006; 12(45): 7319-7325
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7319
Hepatocellular carcinoma (HCC) is one of the most common neoplasms and its incidence is currently rising worldwide[1-3]. HCC usually occurs in cirrhotic livers and less than 30% of patients presenting with HCC are considered candidates for resection[4]. When liver transplantation (LT) was first developed, it was hoped that it would provide a cure for patients with unresectable HCC. However, it became apparent that both intra- and extra-hepatic post-transplant recurrence were frequent, accounting for poor mid-term survival[5-9]. Subsequently, some predictors of recurrence were identified[10-13] in order to select those who may benefit from LT, among patients with HCC.
During the past decade, liver transplantation criteria for HCC were refined and are currently based on the number and size of tumors[10,13]. Other predictors of recurrence have been more recently proposed[14-20]. Some of them, such as micro-vascular involvement[14-18] cannot be assessed prior to LT. Others, such as bi-lobar distribution of HCC[14,18] the sum of diameters of all tumors[19] or tumor differentiation[15-17,19-20] could be evaluated preoperatively and used in clinical practice. In addition, the impact of post transplant factors on HCC recurrence, such as the intensity and type of immunosuppression, have been little studied[21].
The aim of this study was, therefore, to assess in a large series of patients transplanted for HCC, the prognostic value of recurrence criteria, including those more recently proposed, as well as variables that could be evaluated pre-operatively or controlled post-operatively.
Patients transplanted for HCC between 1988 and 1998 in 14 French liver transplant centers were studied. Patients with incidental HCC, which were defined as tumors diagnosed on the explanted liver but not prior to transplant, were excluded.
Based on these criteria, a cohort of 467 patients was identified. Fifty-five (11.8%) patients who died postoperatively and were not exposed to the risk of recurrence were excluded from the analysis of factors associated with recurrence. Therefore, the final study population consisted of 412 patients who had been transplanted for HCC and were suitable for analysis of recurrence and tumor-free survival. The features of these 412 patients are shown in Table 1.
| Age (yr) | 52.7 ± 9.2 |
| Sex (M/F), n (%) | 339 (82.3)/73( 17.7) |
| Etiology: Alc./Vir./Metab./Miscel1, n (%) | 136 (33.2)/236 (57.3)/ 8(2.0)/32 (7.5) |
| Cirrhosis (yes/no), n (%) | 377 (91.5)/35 (8.5) |
| AFP (μg/L) | 26.9 (ext: 0.5-245.240) |
| Number of nodules | 1.8 ± 1.3 (median: 1, range: 1-11) |
| Maximum diameter (cm) | 4.1 ± 3.1 (median: 3.4, range: 0.6-25) |
| Bilobar location (yes/no), n (%) | 105 (25.5)/307 (74.5) |
| Portal or hepatic vein obstruction (yes/no) (n) | 33 (8.1)/379 (91.9) |
| Milan Criteria (yes/no), n (%) | 263 (63.8)/149(36.2) |
| Peri-operative treatment (yes/no), n (%) | 275 (66.8)/137(33.2) |
| Transarterial chemoembolization (yes/no) | 161 (39.2)/251 (60.8) |
| Pre-transplant surgery (yes/no) | 42 (10.2)/370(89.8) |
| Pre-transplant ethanol injection (yes/no) | 65 (15.8)/347 (84.2) |
| Post-transplant chemotherapy (yes/no) | 57 (13.9)/355 (86.1) |
| Tumor differentiation (Well/moderate/poor), n (%) | 248 (69.3)/90 (25.4)/20 (5.3) |
| Time on waiting list (mo) | 4.4 ± 4.0 (median: 3.3, range: 0.1-32.3) |
| Initial immunosuppression Calcineurine inhibitors/ATG-OKT32, n (%) | 357 (86.6)/55 (13.4) |
| Steroid-treated rejection episodes, n (%) | 125 (30.5) |
| Maintenance immunosuppression | |
| Cyclosporine A/tacrolimus, n (%) | 284 (68.9)/128 (31.1) |
Charts of the 412 patients were individually reviewed and the following data were collected.
Pretransplant data: Demographics, etiology and severity of liver disease, liver biochemical tests and prothrombin time, α fetoprotein level, Karnofsky index[22], pretransplant treatments of HCC during the waiting time and time from evaluation to LT (months) were noted. Cirrhotic patients were classified according to Child-Pugh classification[23].
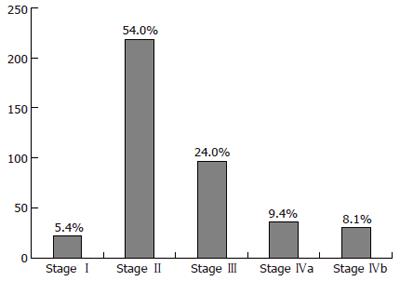
Morphological features and preoperative staging of HCC were evaluated from medical reports of abdominal imaging including ultrasonography, angiography, dynamic computed tomography, and contrast-enhanced magnetic resonance imaging, when available. The number, size, and locations of the tumors as well as the presence of a vascular obstruction were assessed. When the number and size of the nodules differed between the different imaging techniques, the technique that showed the largest number of nodules and the largest nodule was taken into account. From their preoperative characteristics, HCCs were classified according to the modified TNM staging classification for liver transplantation[24] (Figure 1) and according to the Milan Criteria[13] (Table 1).
Treatments of HCC during the waiting time were also assessed [pre-operative treatment: surgery, transarterial chemoembolization (TACE) or percutaneous ethanol injection (PEI)], as well as the immediate post-operative treatments (Table 1).
Pathological study of the tumors: Results of pretransplant tumor biopsies were available in 79 patients. Tumor differentiation was therefore determined by reviewing the pathological reports of the explanted livers, to obtain a larger set of data. Tumor differentiation was graded in 3 stages (grade 1: well-differentiated tumors, grade 2: moderately differentiated tumors and grade 3: poorly differentiated tumors) according to the modified Edmondson Criteria[25]. In case of several tumors showing different stages of differentiation on the explanted liver, the worst grade of differentiation was arbitrarily chosen. Tumor differentiation was obtained in 358 patients (85.4%). It could not be obtained in 40 patients because of total necrosis of tumor nodules due to pre-LT treatment (transarterial chemoembolization or percutaneous ethanol injection), and was not available in 14 patients. Other pathological features which could not be assessed pre-operatively, such as microvascular invasion, number and size of tumor nodules on the explanted liver were also available but were deliberately not taken into account in the present study.
Posttransplant data: The type of immunosuppressive drugs (calcineurin inhibitors vs antilymphocyte antibodies) (Table 1) and the presence of a histologically proven acute rejection and its treatment were noted (methyl-prednisone pulses, antilymphocyte antibodies). Postoperative death was defined as death occurring during the first 3 mo post-LT. Causes of death (deaths due to HCC recurrence and other late causes of deaths), HCC recurrence, length of follow-up from listing on the waiting list to transplantation and from transplantation to death, HCC recurrence or the most recent information, were determined. Data on immunosuppression and adjuvant postoperative treatment of HCC were also collected. As previously described, patients were screened for tumor recurrence by AFP assay and thoracic and abdominal CT every 3 mo for the first two years and/or when clinically indicated. Additional imaging techniques (bone scan, MRI) were used if necessary.
The proportion of missed data ranged from 0.0% to 14.6%, from one variable to another. In the case of missing data, no extrapolation of the missed values was done for the purpose of statistical analysis. Baseline patient characteristics and other continuous variables were reported as means ± SD or median and range when appropriate. Distributions of categorical variables are expressed as percentages. The Kaplan-Meier method was used to evaluate the probability of survival. Kaplan Meier estimates were calculated for 24 variables with potential prognostic significance and compared by the Logrank test. Factors associated with tumor-free survival at a P level of 0.1 in univariate analysis were entered in a multivariate analysis, using a Cox proportional hazards model to identify independent predictors of recurrence.
The median waiting time from evaluation to LT was 3.3 mo (range: 0.1-32.3 mo). Median post-operative follow-up period was 52.0 mo (range: 3.2-186.3 mo). Tumor recurrence occurred in 131 cases (31.8%), after a median of 11.8 mo (1-125 mo). Recurrence involved a single site in 55.1% of the cases and multiple locations in 44.9% of the cases. Recurrence involved the liver graft, lungs, bones, brain, skin and other sites in 49.1%, 44.9%, 34.7%, 6.7%, 5.9% and 14.4% of the cases, respectively. The median time from recurrence to death was 5.6 mo (0.2-62.7 mo). One hundred and ninety-seven patients died (47.8%) during follow-up beyond the postoperative phase. Causes of death were HCC recurrence in 121 cases (61.4%), infections in 14 cases (7.1%), cardiovascular events in 11 cases (5.6%), recurrence of underlying liver disease in 8 cases (4.1%), and de novo cancers in 9 cases (4.5%). By the end of follow-up, only 10 patients with recurrence were alive.
Overall 5-year tumor-free survival was 57.1% (Figure 2). Five-year overall survival was 57.9% ± 2.5% since the vast majority of patients with recurrence died within 6 mo following recurrence. When restricted to the 330 patients transplanted after 1991, 5-year overall survival was 65% ± 2.7%. However, if the database was restricted to these 330 patients, statistical analysis of factors associated with recurrence-free survival did not change.
Among the 24 variables that were tested, 16 variables were associated with tumor-free survival by univariate analysis (Table 2): (1) period of LT (< 1991, 1991 to 1993, 1994 to 1996 and >1996; P < 0.0001). (2) pre-operatively: presence of cirrhosis (P = 0.001), etiology of liver disease (P = 0.03), α fetoprotein level (< 200, 200 to 2000 or > 2000; P < 0.0001), G-GT activity (N, N to 2N or > 2N; P = 0.02), the number of nodules (1, 2-3 or ≥ 4; P = 0.02), maximal diameter of the largest nodule (< 3 cm, 3 to 5 cm or > 5 cm; P < 0.0001), the sum of the diameter of the nodules (< 3 cm, 3 to 5 cm, 5 to 10 cm or > 10 cm; P < 0.0001), bi-lobar location (P = 0.01), preoperative portal or hepatic vein obstruction (P < 0.0001), use of a peri-operative adjuvant treatment (P = 0.002), use of pre-operative transarterial chemoembolization (P = 0.03), (3) pathological data: tumor differentiation (well, moderate or poorly; P = 0.01), (4) post-operatively: the initial type of calcineurin inhibitor (cyclosporine A vs tacrolimus, P = 0.003), the use of antilymphocyte antibodies (mono- and polyclonal) (P = 0.02) and rejection episodes requiring steroid pulses (P = 0.003). It is noteworthy that calcineurin tacrolimus was more recently used in recent cases and that ATG-OKT3 was not routinely used recently.
| Variables (n) | 5-yr recurrencefree survival (%) | P |
| Age < 50 (151)/> 50 (261) | 58.2/56.4 | 0.58 |
| Sex Male (339)/female(73) | 56.8/58.3 | 0.46 |
| Liver disease etiology | ||
| Alcohol (122)/virus (210)/ Alcohol+virus (41)/others (39) | 64.8/55.1/58.1/40.2 | 0.03 |
| Cirrhosis Present (377)/absent (35) | 58.7/35.7 | 0.001 |
| AFP (μg/L) | ||
| < 200 (281)/200-2000 (73)/> 2000 (33) | 62.9/47.8/26.4 | < 0.0001 |
| ASAT | ||
| Normal (139)/N-2N (129)/> 2N (112) | 59.2/60.5/51.1 | 0.3 |
| ALAT | ||
| Normal (178)/N-2N (116)/> 2N (86) | 56.4/63.5/60.6 | 0.3 |
| Alkaline phosphatase | ||
| Normal (170)/high (200) | 60.9/61.1 | 0.2 |
| G-GT | ||
| Normal (107)/N-2N (113)/> 2N (145) | 66.9/59.9/49.7 | 0.02 |
| Child-Pugh classification | ||
| A (219)/B (125)/C (56) | 58.7/49.6/71.1 | 0.07 |
| Karnofsky index | ||
| > 80% (251)/< 80% (155) | 58.2/55.7 | 0.78 |
| Number of nodules, pre- transplant | ||
| 1 (228)/2 or 3 (142)/> 4 (33) | 58.4/58.4/48.5 | 0.02 |
| Maximum diameter of the largest nodule, pre-transplant | ||
| < 3 cm (126)/3-5 cm (191)/> 5 cm (82) | 69.3/61.4/32.9 | < 0.0001 |
| Sum of tumor diameter (cm) | ||
| < 3 cm (123)/3-5 cm (121)/ 5-10 cm (92)/> 10 cm (34) | 72.1/61.1/50.3/19.5 | < 0.0001 |
| Tumor location, pre- transplant | ||
| Uni-lobar (303)/Bi-lobar (104) | 60.4/48.3 | 0.01 |
| Portal or hepatic vein obstruction, pre-transplant | ||
| Absent (373)/Present (33) | 60.2/27.3 | < 0.0001 |
| Pre or post transplant adjuvant treatment | ||
| Present (274)/absent (136) | 61.1/47.9 | 0.002 |
| Pre LT arterial chemoembolization | ||
| Present (241)/absent (162) | 62.3/53.0 | 0.03 |
| Tumor differentiation | ||
| Well (248)/moderate (91)/poor (19) | 58.3/47.1/42.1 | 0.01 |
| Period of transplantation | ||
| < 91 (82)/91-93 (91)/94-96 (118)/97-98 (121) | 26.8/56.0/61.6/73.3 | < 0.0001 |
| Waiting time | ||
| < 6 mo (318)/6-12 mo (71)/> 12 mo (23) | 55.4/67.1/52.2 | 0.18 |
| Maintenance immunosuppression | ||
| Cyclosporine A (264)/tacrolimus (119) | 52.5/70.8 | 0.003 |
| Steroid-treated rejection | ||
| Yes (125)/no (284) | 48.5/60.8 | 0.003 |
| Use of mono or polyclonal antilymphocyte antibodies | ||
| Present (55)/absent (356) | 45.4/58.8 | 0.02 |
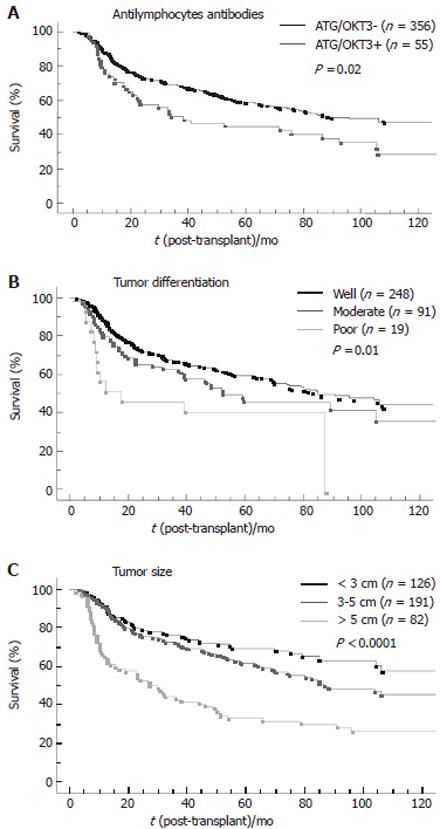
By multivariate analysis (Table 3), 6 variables were independently associated with HCC recurrence: maximal diameter of the largest nodule (P < 0.0001; RR = 1.12), period of LT (P < 0.0001; RR = 0.66), tumor differentiation (P < 0.0001; RR = 1.6), the use of anti-lymphocyte antibody (ATG) or anti-CD3 antibody(OKT3) (P = 0.005; RR = 1.8), preoperative portal or hepatic vein thrombosis (P = 0.06; RR = 1.65) and the number of nodules (P = 0.06; RR = 1.13). Figure 3 shows the influence of the use of anti-lymphocyte antibodies, tumor size and number of nodules on tumor-free survival.
| Relative risk | 95% CI | P | |
| Use of anti-lymphocyte antibodies | 1.8 | 1.2-2.6 | 0.005 |
| Tumor differentiation | 1.6 | 1.24-2.06 | 0.0006 |
| Maximum diameter of the largest nodule | 1.12 | 1.08-1.17 | < 0.0001 |
| Portal/hepatic vein obstruction | 1.6 | 1.01-2.72 | 0.06 |
| Number of nodules | 1.13 | 1-1.28 | 0.06 |
| Recent period of transplantation | 0.66 | 0.54-0.82 | 0.0001 |
This multicenter retrospective study reports one of the largest numbers of patients transplanted for HCC. In this work, we chose to focus on data that could be assessed preoperatively or monitored postoperatively, because such parameters are the only one of clinical relevance in predicting recurrence. On this basis, first, we show that the immunosuppressive regimen, through the use of anti-lymphocyte antibodies, have an independent negative impact on HCC recurrence. Secondly, we confirm that tumor differentiation, a feature that could be assessed preoperatively, has an independent prognostic value. Thirdly, we show that, among the more recently proposed prognostic factors, bi-lobar distribution of the tumor and the sum of diameter of the tumors are not independent predictors or recurrence. Lastly, we obviously re-identify the prognostic value of the number and size of the nodules as well as that of tumorous macrovascular invasion.
The negative impact of anti-lymphocyte antibodies on tumor recurrence has not been reported previously. A negative role of immunosuppression on recurrence had been suggested by Pittsburgh’s group by comparing the doubling time of tumor recurrence after LT or surgical resection[26]. This negative effect was also suggested in a model of tumor recurrence in immunosuppressed, liver transplanted rats[27] and in a model of liver tumor in cyclosporine A-treated rats[28]. More recently it has also been proposed in a clinical study that tumor recurrence could be favored by the intensity of immunosuppression[21]. In this study, the risk of recurrence was proportional to cyclosporine A blood levels. Anti-lymphocyte antibodies are currently the most powerful immunosuppressive agents and could further impair the anti-tumoral mechanisms required to control tumor cell spreading after LT for HCC. This was suggested by the faster recurrence observed during the first 2 years postoperatively in anti-lymphocyte antibodies-treated patients (Figure 3). Caution is therefore mandatory in using anti-lymphocyte antibodies after LT for HCC. This finding also suggests that the clinical studies carried out in the early 1990s, when the criteria for transplantation of hepatocellular carcinoma were introduced, may have been influenced by the immunosuppressive regimens adopted at that time. Since the current trend is to use less powerful immunosuppressive regimens than a decade ago, it raises the possibility that the impact of the current selection criteria on recurrence, based on tumor staging, might have changed over time and should be re-evaluated on a prospective basis[21].
In our study, tumor differentiation had a strong independent value in predicting recurrence: the poorer the differentiation, the higher (Table 3) and faster (Figure 3) the recurrence. This result is in agreement with the results of 3 other groups[15-17,19], which have found an association between tumor differentiation and the risk of recurrence. The negative impact of dedifferentiation could be explained by the relationship between poorly differentiated tumor cells and microvascular invasion, especially in large tumors[17]. In the present study, tumor differentiation was assessed on the explanted liver in order to make use of a larger set of data since the result of preoperative tumor biopsies were available in only 79 cases, and concerned mainly with well-differentiated tumors. These results suggest, however, that grading tumor differentiation could be useful in improving the selection of liver transplant candidates for HCC, provided there is a relationship between tumor grading on preoperative needle biopsy and postoperative grading on the explanted liver. This could be particularly important in the case of large tumors, exceeding 5 cm[17], for which an expansion of the classical criteria of LT has been recently proposed[19]. The presence of poorly differentiated tumor cells in preoperative biopsy samples would argue against LT in such patients, but LT could be considered in a group of patients suffering from well-differentiated tumors. Such an approach is supported by the results of a recent Italian study[29] reporting a 6% rate of recurrence after LT for HCC in patients selected on the basis of a preoperative tumor biopsy showing no poorly differentiated cells and including 38% patients who did not meet the Milan Criteria. Such a strategy would mean a change of current protocols, because pre-transplantation biopsies are generally not obtained as a result of the lack of an obvious benefit and the occasional report of needle-tract seeding[30,31].
The number and size of HCC have been identified a decade ago as 2 major predictors of recurrence. Based on this finding, Mazzafero et al[13] proposed to restrict LT indications to patients with a single nodule < 5 cm in diameter or with < 3 nodules with a maximum diameter of 3 cm to minimize post-LT recurrence. More recently, bi-lobar distribution of the tumor and the sum of diameter of the tumors, 2 factors that take into account size and number, have been proposed as predictors of recurrence[14,18,19]. In the present series, we found that these two last factors had no independent predictive value. With respect to this point, we must point out that in our multivariate analysis, the number of tumors was the factor that had the lowest power in predicting recurrence with a P value at 0.06; the lack of independent value of bi-lobar distribution, which can be considered a peculiar form of multifocal HCC, is therefore not unexpected. In addition, our results indicate that the size of the largest nodule is more closely related with recurrence than the sum of diameters of all tumors, suggesting that the sum of diameters of the nodules is mainly determined by the size of the largest nodule in a majority of cases. According to the poor recurrence-free survival of patients with a maximal diameter of the largest nodule exceeding 5 cm, we actually consider that liver transplantation should be avoided in such cases.
Finally, we found that a recent period of LT was independently associated with tumor-free survival. This suggests that other factors than those identified in the present study that may have varied over time, such as maintenance immunosuppression levels[21], or improvements in surgical techniques, limiting the peri-operative risk of tumor cell spreading, might also influence recurrence.
In conclusion, we have identified the use of anti-lymphocyte antibodies as a new predictive factor of tumor recurrence after LT for HCC. We also confirmed the independent prognostic value of tumor differentiation and did not confirm the independent prognostic value of either bi-lobar distribution of the tumor or the sum of diameters of the tumors. Based on these results we propose to avoid the use of anti-lymphocyte antibodies after transplantation for HCC, a finding that also raises the issue of a change in the impact of tumor staging over time on recurrence, due to parallel changes in immunosuppression policies. We also propose to test prospectively the usefulness of a pre-transplant tumor biopsy in order to evaluate the importance of tumor differentiation in the decision-making algorithm of candidates for transplantation in HCC.
| 1. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 2. | Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 3. | Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997;350:1142-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Olthoff KM. Surgical options for hepatocellular carcinoma: resection and transplantation. Liver Transpl Surg. 1998;4:S98-104. [PubMed] |
| 5. | Iwatsuki S, Gordon RD, Shaw BW Jr, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 354] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | O'Grady JG, Polson RJ, Rolles K, Calne RY, Williams R. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 330] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Ringe B, Wittekind C, Bechstein WO, Bunzendahl H, Pichlmayr R. The role of liver transplantation in hepatobiliary malignancy. A retrospective analysis of 95 patients with particular regard to tumor stage and recurrence. Ann Surg. 1989;209:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 266] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Ismail T, Angrisani L, Gunson BK, Hübscher SG, Buckels JA, Neuberger JM, Elias E, McMaster P. Primary hepatic malignancy: the role of liver transplantation. Br J Surg. 1990;77:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 420] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 632] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 11. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-28; discussion 221-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 471] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | McPeake JR, O'Grady JG, Zaman S, Portmann B, Wight DG, Tan KC, Calne RY, Williams R. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5388] [Article Influence: 179.6] [Reference Citation Analysis (7)] |
| 14. | Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, Subbotin V, Casavilla A, Carr BI, Fung JJ. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology. 1997;26:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 296] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 16. | Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: an update of the International Tumor Registry. Liver Transpl. 2002;8:736-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 710] [Article Influence: 28.4] [Reference Citation Analysis (1)] |
| 18. | Iwatsuki S, Dvorchik I, Marsh JW, Madariaga JR, Carr B, Fung JJ, Starzl TE. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 20. | Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25-30; discussion 31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, La Barba G, Brillanti S, Cavallari A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression. Transplantation. 2002;74:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Karnofsky DA. Palliation and life prolongation with anticancer drugs. CA Cancer J Clin. 1966;16:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5821] [Article Influence: 109.8] [Reference Citation Analysis (2)] |
| 25. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 26. | Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Ceriello A, Mezza F, Cozzolino S, Pettinato G, Mancini A, Santaniello W, Calise F, Cuomo O. Role of immunosuppression in recurrence after liver transplantation for diethylnitrosamine-induced tumors in rats. Transpl Int. 1994;7 Suppl 1:S204-S207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Freise CE, Ferrell L, Liu T, Ascher NL, Roberts JP. Effect of systemic cyclosporine on tumor recurrence after liver transplantation in a model of hepatocellular carcinoma. Transplantation. 1999;67:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 30. | Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary. Liver Transpl. 2000;6:67-72. [PubMed] |
| 31. | Chapoutot C, Perney P, Fabre D, Taourel P, Bruel JM, Larrey D, Domergue J, Ciurana AJ, Blanc F. [Needle-tract seeding after ultrasound-guided puncture of hepatocellular carcinoma. A study of 150 patients]. Gastroenterol Clin Biol. 1999;23:552-556. [PubMed] |
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L