Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7263
Revised: October 28, 2006
Accepted: November 9, 2006
Published online: December 7, 2006
AIM: To investigate the effect of troglitazone on pe-roxisome proliferator-activated receptor γ (PPARγ) expression and cellular growth in human colon cancer HCT-116 and HCT-15 cells and to explore the related molecular mechanism.
METHODS: Human colon cancer HCT-116 and HCT-15 cells cultured in vitro were treated with troglitazone. Reverse transcription-polymerase chain reaction (RT-PCR) and Western blot were employed to detect the effect of troglitazone on PPARγ expression. The proliferative activity was determined by MTT assay, cell cycle and apoptosis were detected by flow cytometry. Apoptosis-related genes, cell cycle regulatory genes and p53 were examined by RT-PCR and Western blot respectively.
RESULTS: The expression of PPARγ in colon cancer HCT-116 and HCT-15 cells was up-regulated by troglitazone. Troglitazone inhibited proliferation, induced apoptosis and cell cycle G1 arrest in colon cancer cells. Troglitazone induced p53 expression in HCT-116 cells, but not in HCT-15 cells. The down-regulation of survivin and bcl-2 was found in both cell lines and up-regulation of bax was found only in HCT-116 cells, being consistent with growth inhibition in HCT-116 cells but not in HCT-15 cells. Troglitazone increased expression of p21WAF1/CIP1 (p21), p27KIP1 (p27) and reduced cyclin D1 in HCT-116 cells while only a minor decrease of cyclin D1 was found in HCT-15 cells.
CONCLUSION: Troglitazone is an inductor of PPARγ in colon cancer cells and inhibits PPARγ-dependently proliferation, which may attribute to cell cycle G1 arrest and apoptosis in colon cancer cells. Troglitazone may induce p53-independent apoptosis and p53-dependent expression of p21 and p27. Depending on cell background, different activation pathways may exist in colon cancer cells.
- Citation: Ming M, Yu JP, Meng XZ, Zhou YH, Yu HG, Luo HS. Effect of ligand troglitazone on peroxisome proliferator-activated receptor γ expression and cellular growth in human colon cancer cells. World J Gastroenterol 2006; 12(45): 7263-7270
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7263
Nowadays, there is increasing interest in the use of specific agonists of peroxisome proliferator-activated receptor γ (PPARγ) as a new antineoplastic approach. PPARγ is a member of the nuclear receptor superfamily of ligand-dependent transcriptional factors[1-3]. It has been shown that PPARγ plays an important role in the differentiation of adipocytes and monocytes/macrophages, as well as in cell proliferation, apoptosis and carcinoma cell arrest[4-6]. Many investigators have focused on the role of PPARγ in colon cancer because PPARγ is highly expressed in human colon and colorectal cancer[4,5].
PPARγ is an excellent target for cancer chemotherapy because it expresses highly in tumors and its activation results in decreased cell proliferation and progression from G0-G1 to S phase, increased differentiation and apoptosis. However, within each cancer type, individual cell lines have been found to respond differently to distinct PPARγ ligands according to ligand structure and cell context. For example, PPARγ-activated C-DIMs could induce p21 expression in Panc-28 but not in other pancreatic cell lines[7]. Rosglitazone, ciglitazone and PGJ2 are all potent agonists of PPARγ transactivation in lung adenocarcinoma cell lines but have no effect on squamous cell or large cell carcinomas of the lung[8]. It is somewhat paradoxical about the induction of these responses by PPARγ agonists and the relative contributions of these pathways are often not well-defined. The PPARγ ligand, troglitazone, one of the thiazolidinediones which are used in the treatment of type II diabetes, is a potent and selective activator of PPARγ. The role of troglitazone in growth of cancer cells has been elucidated in some studies[3,9]. It has been shown that troglitazone has negative effects on the proliferation of malignant tumor cells, including colon cancer cells[3]. However, Lucarelli et al[9] found that troglitazone could stimulate growth of osteosarcoma cells. Up to now, whether troglitazone inhibits or stimulates the growth of cancer cells is still unclear. Whether troglitazone activates PPARγ-dependent and/or –independent pathways, which may be beneficial for cancer chemotherapy, has not been elucidated.
This study was to detect the effect of troglitazone on PPARγ expression and colon cancer cell growth and to explore its molecular mechanism. In order to elucidate the effect of troglitazone on colon cancer cells, we chose colon cancer HCT-116 and HCT-15 cells, because these two cell lines are wild-type (WT) and mutant-type p53 respectively[10].
Two established human colon cancer cell lines, HCT-116 and HCT-15 (Central Chinese Type Culture Collection, Wuhan, China) were used in this study. All standard culture reagents were obtained from Gibco BRL Inc., USA. Troglitazone was obtained from Alexis Corporation (Switzerland) and dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 0.1% DMSO in the culture medium. 3, [4, 4-dimethylthiazol-2-yl] 2, 5 diphenyltetrazolium bromide (MTT) was purchased from Serva (Germany). Annexin V-FITC and TRIzol were obtained from Pharmingen and Invitrogen (USA). Anti-PPARγ, anti-p53, anti-p21, anti-p27, anti-cyclin D1, anti-survivin, anti-bcl-2, anti-bax and anti-β-actin were purchased from Santa Cruz Biotechnology (USA). All primers were obtained from Shanghai Sangon Biotechnology (China).
HCT-116 and HCT-15 cells were maintained in Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 0.238% N-2-hydroxy-ethlpiperazine-N-2-ethanesulfonic acids (HEPES), 100 U/mL penicillin G and 0.1 mg/mL streptomycin. Cells were incubated at 37°C in the 5% CO2 incubator. Before the experiment, the cells were incubated in a serum-free medium for 12 h and treated with experimental reagents.
Cell proliferation was evaluated by MTT assay. Briefly, cells were seeded at a density of 5000 cells/well in 96-well plates with or without troglitazone. DMSO was used as vehicle control. MTT solution in phosphate-buffered saline (PBS) was added to reach a final concentration of 0.5 mg/mL and incubated for 4 h at 37°C. Medium was removed and cells were resuspended in 100 μL DMSO, and then the absorbance was measured at 570 nm using a microplate reader. The ratios of the absorbance of treated cells relative to those of control wells were calculated and expressed as proliferation percentage.
The cells were collected by centrifugation and washed with PBS. The pellets were resuspended in ice cold 70% ethanol and fixed at 4°C for 24 h. The cells were centrifuged and washed repeatedly with PBS for removal of ethanol. The cell pellets were resuspended in 1 mL DNA staining reagent and kept at 4°C for 30 min. DNA contents were analyzed using a FACS 440 flow cytometer (Becton Dickinson). Apoptosis was evaluated by Annexin V-FITC and flow cytometer. Cells were harvested and washed with PBS, and then stained with Annexin V-FITC and 2 μg/mL PI in binding buffer for 15 min at 37°C in the dark. Finally, the samples were analyzed by using a FACS 440 flow cytometer.
Total RNA was isolated from human colon cancer cells with TRIzol reagent following the manufacturer’s instructions. Concentration of the RNA was detected by the absorbance at 260 nm, and the integrity was verified by electrophoresis on formaldehyde gels. RT-PCR was carried out as described previously[11]. Total RNA was reverse-transcribed into complementary deoxyribonucleic acid (cDNA) which was subjected to PCR for measurement of messenger RNA (mRNA). The product of PCR was checked by 2% agarose gel electrophoresis for a single band of the expected size. The abundance of each mRNA was detected and normalized to that of GAPDH mRNA. The sequences of all primers used in this project are shown in Table 1.
| Genes | Primer sequences (5’-3’) | PCR products (bp) |
| PPARγ | Forward CTCTCCGTAATGGAAGACC | 474 |
| Reverse GCATTATGAGACATCCCCAC | ||
| P21 | Forward GCGATGGAACTTCGACTTTGA | 354 |
| Reverse GGGCTTCCTCTTGGAGAAGAT | ||
| P27 | Forward AATAAGGAAGCGACCTGCAA | 451 |
| Reverse CCTCCCTTCCCCAAAGTTTA | ||
| Cyclin D1 | Forward GGCAACGGAGGTCTGCG | 323 |
| Reverse GTCGGTGGTAGATGCACAGCTT | ||
| Survivin | Forward CACCGCATCTCTACATTCAA | 345 |
| Reverse CACTTTCTTCGCAGTTTCCT | ||
| Bcl-2 | Forward GGCAATGTGACTTTTTCCAA | 137 |
| Reverse GGCTGATATTCTGCAACACTG | ||
| Bax | Forward CCAGCTGCCTTGGACTGT | 135 |
| Reverse ACCCCCTCAAGACCACTCTT | ||
| GAPDH | Forward CCATGGAGAAGGCTGGGG | 200 |
| Reverse CAAAGTTGTCATGGATGACC |
Nuclear and cytoplasmic extractions were carried out as previously described[12]. Protein concentrations were measured with a Micro BCA protein assay reagent kit. After being boiled in electrophoresis SDS sample buffer, 40 μg proteins were separated on 10% polyacrylamide gel and transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked with blocking buffer (5% fat-free skimmer milk with 0.1% Tween 20) for 2 h. Subsequently, the membranes were washed with TBST buffer and incubated with the particular primary antibodies diluted in the blocking buffer overnight at 4°C. Then the membranes were washed with TBST buffer and incubated with secondary antibodies. The proteins were detected by enhanced chemiluminescence reagent according to its manufacturer’s instructions. The abundance of each protein was detected and normalized to that of β-actin.
All experiments were performed in triplicate and the data were expressed as mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA) using SPSS11.0 for windows and P < 0.05 was considered statistically significant.
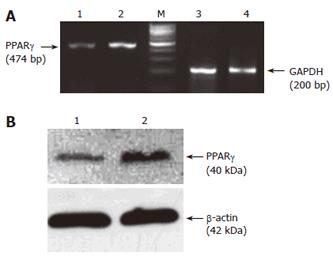
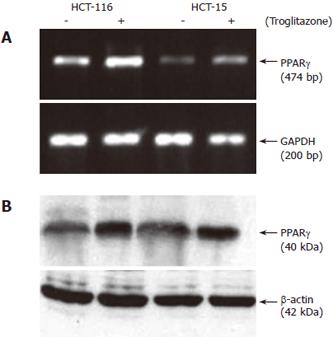
The expression of PPARγ in colon cancer cells was detected by RT-PCR and Western blot. Before exposure to troglitazone, PPARγ was expressed in both HCT-116 and HCT-15 cells, while the mRNA and protein levels of PPARγ in the former were higher than those in the latter (Figure 1A and 1B). After cells were treated with 10 μmol/L troglitazone, the mRNA level of PPARγ increased by 3.03-fold (3.12 ± 0.55 vs 1.03 ± 0.31, P < 0.01) and 1.52-fold (1.02 ± 0.35 vs 0.67 ± 0.25, P < 0.05) (Figure 2A) and the protein level increased by 1.86-fold (2.33 ± 0.53 vs 1.25 ± 0.42, P < 0.05) and 1.48-fold (1.95 ± 0.48 vs 1.32 ± 0.38, P < 0.05) respectively (Figure 2B).
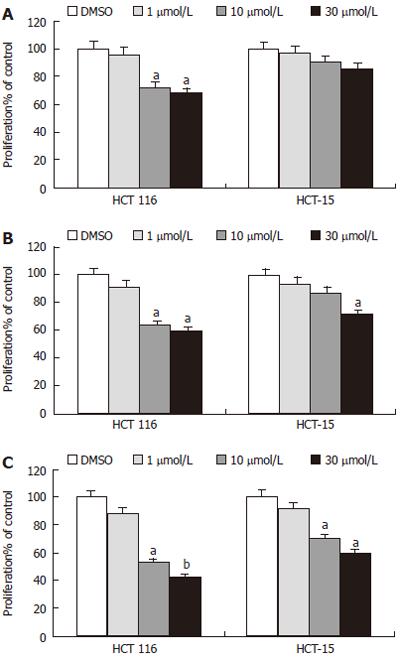
MTT assay was used to determine the effect of troglitazone on growth of colon cancer. Two cell lines were treated with different concentrations (1, 10 and 30 μmol/L) of troglitazone for different periods of time (12, 24 or 48 h). The results illustrated that troglitazone inhibited significantly proliferation of both HCT-116 and HCT-15 cells in a concentration- and time-dependent manner, which were observed as early as 12 h in HCT-116 cells and 48 h in HCT-15 cells after treatment with 10 μmol/L troglitazone. Significant proliferation inhibition of HCT-15 cells could be only found at 24 h with 30 μmol/L troglitazone (Figure 3).
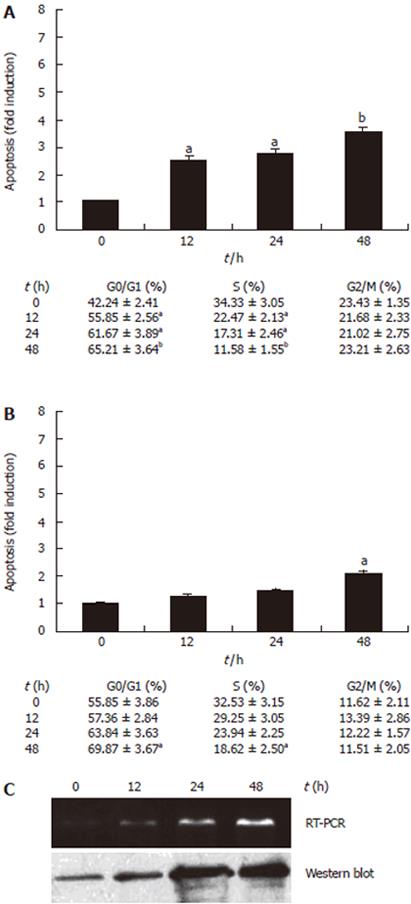
To determine whether the decrease in cell proliferation was associated with cell cycle arrest or apoptosis, cells were treated with 10 μmol/L troglitazone for 12, 24 or 48 h, then cell cycle and apoptosis were analyzed by using a flow cytometer. Ten μmol/L troglitazone led to increase in percentage of cells in G1 phase and decrease in S phase at 12 h in HCT-116 cells (Figure 4A) and at 48 h in HCT-15 cells (Figure 4B), and there was no significant change in the proportion of cells in G2/M phase. Moreover, the apoptosis rate for HCT-116 cells increased at 12 h (2.52-fold) while that for HCT-15 cells increased at 48 h (2.09-fold), albeit the apoptosis rate gradually increased time-dependently in both cell lines (Figure 4). These results were consistent with the growth inhibition of HCT-116 and HCT-15 cells (Figure 3), suggesting that the anti-proliferation effect of troglitazone should be associated with cell cycle G1 arrest and apoptosis in colon cancer cells.
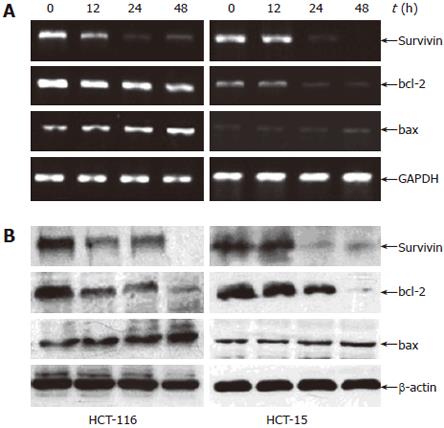
It is well-known that p53 plays an important role in the induction of apoptosis and growth inhibition, so we tried to investigate the potential mechanism by which troglitazone causes apoptosis and to clarify whether p53 is related with troglitazone-induced apoptosis in colon cancer cells. After cells were treated with 10 μmol/L troglitazone for 12, 24 or 48 h, mRNA and protein levels of p53 and apoptosis-related genes, such as survivin, bcl-2 and bax, were analyzed by RT-PCR and Western blot. P53 expression was found to be affected by troglitazone in a time-dependent manner in HCT-116 cells (Figure 4C) but not in HCT-15 cells. As shown in Figure 5A, the mRNA levels of survivin and bcl-2 in HCT-116 and HCT-15 cells were down-regulated at 12 h and 24h, respectively. Moreover, the up-regulation of bax was found only in HCT-116 cells, but not in HCT-15 cells. The protein levels detected by Western blot were consistent with the mRNA levels in the three genes (Figure 5B). After exposure to troglitazone for 24 h, the decreased survivin and bcl-2 expression and the unchanged bax expression in HCT-15 cells without corresponding apoptosis suggested that neither survivin or bcl-2 nor bax might be the key regulators for troglitazone-induced apoptosis in HCT-15 cells.
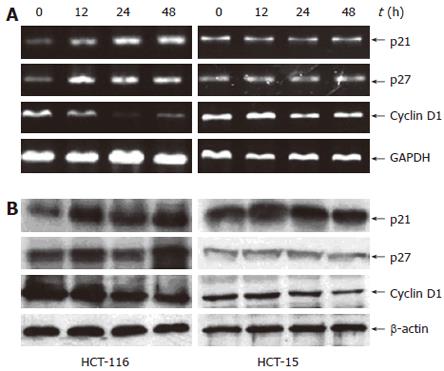
After cells were treated with 10 μmol/L troglitazone, vari-ous markers of cell cycle progression, such as p21WAF1/CIP1 (p21), p27KIP1 (p27) and cyclin D1 were measured at different time points for verifying the effect of troglitazone on cell cycle regulatory genes. It was observed that the effect of troglitazone on the induction of p21 at mRNA and protein levels in HCT-116 cells was time-dependent. The transcriptional up-regulation of p27, another type of cyclin-dependent kinase inhibitor, was also observed. In addition, cyclin D1 expression was also down-regulated in a time-dependent manner (Figure 6). These results were consistent with cell cycle G1 arrest in HCT-116 cells (Figure 4A). Neither p21 nor p27 was found to be significantly altered in HCT-15 cells, but a minor down-regulation of cyclin D1 was observed (Figure 6). The reasons why p21 and p27 in the two cell lines reacted to troglitazone differently were unclear. Troglitazone could not induce p21 or p27 in HCT-15 cells but could induce p21 and/or p27 expression in a p53-dependent manner.
PPARγ ligands have been shown to regulate gene networks involved in control of cell growth, differentiation, apoptosis and cell cycle[1-3]. Structurally diverse PPARγ agonists inhibit the growth of multiple cancer cell lines[7,13-15], including colon cancer cells. However, the mechanism of growth inhibition is dependent on ligand structure and cell context.
This study was therefore undertaken in an effort to provide additional support for the hypothesis that PPARγ is a negative growth regulator of colon cancer cells. The results showed that PPARγ was expressed in colon cancer HCT-116 and HCT-15 cells, which is consistent with previous report that PPARγ was expressed highly in colon cancer cells[15]. The fact that troglitazone treatment resulted in increased PPARγ mRNA and protein levels in both cell lines suggests that PPARγ signaling is functional. Furthermore, the enhancement of PPARγ in HCT-116 cells was higher, indicating that HCT-116 cells may be more sensitive to troglitazone treatment than HCT-15 cells.
The effect of troglitazone on growth of colon cancer cells was determined by MTT assay. Although troglitazone inhibited growth of both cell lines in a concentration- and time-dependent manner, there were significant differences in the responsiveness of colon cancer cells to the treatment with troglitazone. The growth of HCT-116 cells was inhibited significantly at 12 h (Figure 3) by 10 μmol/L troglitazone, which is consistent with that by 5 μmol/L troglitazone reported by Baek et al[16]. In contrast, the growth of HCT-15 cells could be only inhibited by high concentrations of troglitazone (30 μmol/L) at 24 h (Figure 3). A recent study in HCT-15 cells found that only 50 μmol/L troglitazone could induce the same responses, indicating that activation of some (K422Q) PPARγ-dependent mutant responses in HCT-15 cells may be observed with higher concentrations of thiazolidinediones[17]. In our study, troglitazone decreased cell growth in both cell lines, but only high concentration could inhibit growth of HCT-15 cells, suggesting that HCT-15 cells are less responsive to the growth inhibition effect of troglitazone than HCT-116 cells.
There is growing evidence that cell cycle arrest and apoptosis play a key role in the development of human cancer. Troglitazone and other PPARγ-modulating drugs are known to induce cell cycle G1 arrest and apoptosis in some cell types[18]. Our results showed that troglitazone resulted in a remarkable increase of cells in G1 phase and induced apoptosis at 12 h in HCT-116 cells (Figure 4A), but significant increase in G1 phase and induction of the fraction apoptosis were found at 48 h in HCT-15 cells (Figure 4B). This phenomenon was coincident with the growth inhibition in both cell lines. Therefore, the inhibitory effect of troglitazone on cell growth of both colon cancer cell lines may be partly due to cell cycle G1 arrest and apoptosis. Similar effects of troglitazone on other colon cancer cells, liver cancer cells and bladder cancer cells have been reported[3,19,20].
Since troglitazone could inhibit growth and induce G1 arrest and apoptosis in HCT-116 and HCT-15 cells, more attention has been paid to its potential mechanism. Wild type p53 is a potential mediator of apoptosis and more sensitive to DNA-damaging treatment than mutant p53[21]. It is also demonstrated that wild type p53 in human colon epithelial cells is functionally dominant over mutant p53 and restoration of wild type p53 expression is insufficient to trigger apoptosis of transformed colonic cells[22]. The fact that troglitazone induced p53 in HCT-116 cells (wild type p53) but not in HCT-15 cells (mutant p53), along with induction of apoptosis in both cell lines, suggests that troglitazone-induced apoptosis in both cell lines in vitro may be p53-independent. Different results in human gastric cancer MKN-74 cells suggest that p53 might be involved in troglitazone-induced apoptosis[23], which may lie in the different cell context.
Survivin, a member of inhibitors of apoptosis family, plays an important role in cell proliferation and cell death. Down-regulation of survivin may cause a cell-cycle defect that leads to programmed cell death[24]. It has been reported that troglitazone can cause a marked decrease of survivin in glioblastoma cell lines[25]. Other critical regulators of apoptosis, such as bcl-2, bcl-xL (which promotes survival) and bax (which promotes apoptosis), also play a different role in programmed cell death[26-28]. Some researches on PPARγ ligand-induced apoptosis in human myeloid leukemia K562 and HL-60 cell lines showed that up-regulation of bax as well as down-regulation of survivin and bcl-2 may be the important mechanism underlying the induction of apoptosis[26]. However, other reports have shown different mechanisms, depending on the target cell type, PPARγ agonists, duration of treatment, dosage, and the presence of other mitogenic factors[27,28]. In our study, troglitazone decreased survivin and bcl-2 expression and increased bax expression at 12 h in HCT-116 cells, which were consistent with corresponding apoptosis, further suggesting that these apoptosis-associated genes may participate in troglitazone-induced apoptosis in HCT-116 cells. Similar results have also found in breast cancer cells[29] and hepatic cancer HepG2 cells[30]. Troglitazone decreased survivin and bcl-2 expression in HCT-15 cells at 24 h and had no impact on bax expression, which did not correlate with any alteration in troglitazone-induced apoptosis. These data indicate that survivin, bcl-2 and bax do not play a major role in the regulation of troglitazone-induced apoptosis of HCT-15 cells. Chintharlapalli et al[27] have reported that the cell context-dependent differential induction of caveolins 1 and 2 may be connected with this process, which is under further investigation.
Inhibition of cell cycle has been considered a target for the management of cancer. We detected the effect of troglitazone on modulation of p53 and its natural target genes p21, p27 and studied the role of two types of p53 in colon cancer cells by examining their effect on cell cycle regulatory genes. P21 and p27 are the downstream effectors of p53. Generally, p21 regulates CDK2-cyclin complexes at the G1 phase of cell cycle following DNA damage or nucleotide pool perturbation, while p27 is a key regulator of the G1/S phase entry and involves the response of cells to environmental mitogen stimulation[31-33]. Once activated by cellular signals, p53 brings about transcription of several cell-cycle regulatory genes, including p21 and p27[34]. P21 expression can be up-regulated by p53-independent or p53-dependent mechanisms, and mutant type p53 induces lower expression of p21 than non-mutant p53[35,36]. Increased p53 expression and transcription-induced p21 and p27 in response to troglitazone treatment at 12 h in HCT-116 cells were found in our study. However, troglitazone had no significant influence on HCT-15 cells, indicating that p21 and p27 play an important role in cell cycle arrest by suppressing HCT-116 cell proliferation. It might be reasonable to consider that troglitazone-induced p21 and p27 may function in a p53-dependent manner. In addition, cyclin D1 is another major positive regulator of G1-S transition by binding to and activating cyclin-dependent kinase 4 or 6, which then phosphorylates and thereby inactivates the tumor suppressor protein pRB[37]. In our study, troglitazone decreased cyclin D1 expression at mRNA and protein levels in HCT-116 cells but only slightly decreased cyclin D1 expression in HCT-15 cells (Figure 6), demonstrating that p21, p27 and cyclin D1 may play a role in the regulation of troglitazone-induced G1 arrest in HCT-116 cells, which are different from some studies on the other type of PPARγ ligands, such as ciglitazone[38]. Meantime, the difference in activation of specific cell cycle regulatory genes by troglitazone was observed between HCT-116 and HCT-15 cells, indicating that different activation pathways may exist in different cell responses to troglitazone-induced G1 arrest, further investigations are underway to clarify the mechanism.
In conclusion, troglitazone is an inductor of PPARγ, a member of nuclear receptor superfamily of ligand-dependent transcriptional factors that has pro-apoptotic and antitumorigenic properties in colon cancer cells. PPARγ-dependent proliferation inhibited by troglitazone is predominant and the growth inhibition attributes partly to cell cycle G1 arrest and apoptosis. Troglitazone may induce p53-independently apoptosis but p53-dependently expression of p21 and p27. Different activation pathways may exist in colon cancer cells.
The authors are grateful to colleagues in gastroenterology laboratory for their advice and help, and Dr. H Xia for his technical assistance.
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor superfamily of ligand-dependent transcriptional factors. There is increasing interest in the use of specific agonists of PPARγ as a new antineoplastic approach.
PPARγ is an excellent target for cancer chemotherapy because it expresses highly in tumors and its activation results in decreased cell proliferation, decreased G0-G1 to S phases progression, increased differentiation and apoptosis. However, within each cancer type, individual cell lines were found to respond differently to distinct PPARγ ligands according to ligand structure and cell context.
Troglitazone is an inductor of PPARγ in colon cancer cells and it inhibits PPARγ-dependently proliferation, which may attribute to cell cycle G1 arrest and apoptosis in colon cancer cells. Troglitazone may induce p53-independently apoptosis and p53-dependently expression of p21 and p27. Depending on cell background, different activation pathways may exist in colon cancer cells.
To conclude, this results in this article qualify PPARγ ligands as promising antineoplastic agents and form the basis for future PPARγ ligands-mediated therapeutic approaches.
PPARγ: peroxisome proliferator-activated receptor gamma. PPARγ is a member of the nuclear receptor superfamily of ligand-dependent transcriptional factors, It has been showed that PPARγ plays an important role in the differentiation of adipocytes and monocytes/macrophages, as well as in cell proliferation, apoptosis and carcinoma cell arrest.
The main objectives of the study were to determine the exression of PPAR gamma in colon cancer HCT-116 and HCT-15 cells and to focus on the proliferation and apoptosis under the influence of troglitazone. Expression of PPAR gamma was demonstrated by RT-PCR and immunoblotting. Proliferation was evaluated by MTT assay. Cell cycle arrest and apoptosis were measured by immunoblotting and FACS analysis. Involvement of p53 was determined by RT-PCR and genes associated with p53 activation were also measured by immunoblotting (p21, p27 and cyclin D1). Overall this is a complete story providing strong evidences that PPARgamma agonist may be useful in colon cancer therapy. The authors point out that not all cancer cell lines react to these agonists in the same fashion and thus each cell line should be taken under examination.
| 1. | Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 454] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 2. | Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor gamma (PPARgamma) ligand induced apoptosis in colon cancer cells. Gut. 2002;50:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 755] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Brockman JA, Gupta RA, Dubois RN. Activation of PPARgamma leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn J Cancer Res. 1999;90:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor gamma-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;145:5774-5785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Allred CD, Kilgore MW. Selective activation of PPARgamma in breast, colon, and lung cancer cell lines. Mol Cell Endocrinol. 2005;235:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Lucarelli E, Sangiorgi L, Maini V, Lattanzi G, Marmiroli S, Reggiani M, Mordenti M, Alessandra Gobbi G, Scrimieri F, Zambon Bertoja A. Troglitazione affects survival of human osteosarcoma cells. Int J Cancer. 2002;98:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, Lacorte JM, Staedel C, Lesuffleur T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Shen ZN, Nishida K, Doi H, Oohashi T, Hirohata S, Ozaki T, Yoshida A, Ninomiya Y, Inoue H. Suppression of chondrosarcoma cells by 15-deoxy-Delta 12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem Biophys Res Commun. 2005;328:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Li JH, Yu JP, Yu HG, Xu XM, Yu LL, Liu SQ. Expression and significance of nuclear factor kappaB p65 in colon tissues of rats with TNBS-induced colitis. World J Gastroenterol. 2005;11:1759-1763. [PubMed] |
| 13. | Rumi MA, Ishihara S, Kadowaki Y, Ortega-Cava CF, Kazumori H, Kawashima K, Yoshino N, Yuki T, Ishimura N, Kinoshita Y. Peroxisome proliferator-activated receptor gamma-dependent and -independent growth inhibition of gastrointestinal tumour cells. Genes Cells. 2004;9:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Chen YX, Zhong XY, Qin YF, Bing W, He LZ. 15d-PGJ2 inhibits cell growth and induces apoptosis of MCG-803 human gastric cancer cell line. World J Gastroenterol. 2003;9:2149-2153. [PubMed] |
| 15. | Gupta RA, Sarraf P, Mueller E, Brockman JA, Prusakiewicz JJ, Eng C, Willson TM, DuBois RN. Peroxisome proliferator-activated receptor gamma-mediated differentiation: a mutation in colon cancer cells reveals divergent and cell type-specific mechanisms. J Biol Chem. 2003;278:22669-22677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma ) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845-5853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H. Induction of differentiation and peroxisome proliferator-activated receptor gamma expression in colon cancer cell lines by troglitazone. J Cancer Res Clin Oncol. 2004;130:73-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Pandhare J, Cooper SK, Phang JM. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J Biol Chem. 2006;281:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Guan YF, Zhang YH, Breyer RM, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in human transitional bladder cancer and its role in inducing cell death. Neoplasia. 1999;1:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. Peroxisome proliferator-activated receptor gamma ligands inhibit cell growth and induce apoptosis in human liver cancer BEL-7402 cells. World J Gastroenterol. 2003;9:1683-1688. [PubMed] |
| 21. | Wang C, Eshleman J, Lutterbaugh J, Bin Y, Willson J, Markowitz S. Spontaneous apoptosis in human colon tumor cell lines and the relation of wt p53 to apoptosis. Chin Med J (Engl). 1996;109:537-541. [PubMed] |
| 22. | Yang B, Stambrook PJ, Markowitz SD. Wild-type p53 demonstrates functional dominance in a human colon carcinoma cell line in which it induces reversible growth arrest. Clin Cancer Res. 1996;2:1639-1647. [PubMed] |
| 23. | Nagamine M, Okumura T, Tanno S, Sawamukai M, Motomura W, Takahashi N, Kohgo Y. PPAR gamma ligand-induced apoptosis through a p53-dependent mechanism in human gastric cancer cells. Cancer Sci. 2003;94:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Paydas S, Tanriverdi K, Yavuz S, Disel U, Sahin B, Burgut R. Survivin and aven: two distinct antiapoptotic signals in acute leukemias. Ann Oncol. 2003;14:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Schultze K, Böck B, Eckert A, Oevermann L, Ramacher D, Wiestler O, Roth W. Troglitazone sensitizes tumor cells to TRAIL-induced apoptosis via down-regulation of FLIP and Survivin. Apoptosis. 2006;11:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Liu JJ, Huang RW, Lin DJ, Peng J, Wu XY, Lin Q, Pan XL, Song YQ, Zhang MH, Hou M. Expression of survivin and bax/bcl-2 in peroxisome proliferator activated receptor-gamma ligands induces apoptosis on human myeloid leukemia cells in vitro. Ann Oncol. 2005;16:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Chintharlapalli S, Smith R 3rd, Samudio I, Zhang W, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor gamma-mediated growth inhibition, transactivation, and differentiation markers in colon cancer cells. Cancer Res. 2004;64:5994-6001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARgamma-dependent and PPARgamma-independent pathways. Mol Cancer Ther. 2006;5:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Lu M, Kwan T, Yu C, Chen F, Freedman B, Schafer JM, Lee EJ, Jameson JL, Jordan VC, Cryns VL. Peroxisome proliferator-activated receptor gamma agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem. 2005;280:6742-6751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. PPARgamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol. 2003;9:1220-1226. [PubMed] |
| 31. | Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000;436:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Dulić V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1070] [Article Influence: 33.4] [Reference Citation Analysis (6)] |
| 33. | Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4456] [Cited by in RCA: 4533] [Article Influence: 167.9] [Reference Citation Analysis (3)] |
| 34. | Kirsch DG, Kastan MB. Tumor-suppressor p53: implications for tumor development and prognosis. J Clin Oncol. 1998;16:3158-3168. [PubMed] |
| 35. | Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 536] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 36. | Shiohara M, el-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R, Chen DL, Vogelstein B, Koeffler HP. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994;84:3781-3784. [PubMed] |
| 37. | Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689-3695. [PubMed] |
| 38. | Chen F, Harrison LE. Ciglitazone-induced cellular anti-proliferation increases p27kip1 protein levels through both increased transcriptional activity and inhibition of proteasome degradation. Cell Signal. 2005;17:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH