Published online Nov 28, 2006. doi: 10.3748/wjg.v12.i44.7203
Revised: September 28, 2006
Accepted: October 15, 2006
Published online: November 28, 2006
In benign or low-grade malignant pancreatic tumors, complete removal of the lesion is sufficient for a cure, and thus minimal resection techniques with preservation of the pancreatic functional reserve have advantages over more extended pancreatic resections. However, a high incidence of postoperative pancreatic fistula in such procedures has been reported. Moreover, branch-type intraductal papillary mucinous neoplasms of the pancreas tend to locate in the head of the pancreas, and show less malignant potential. We describe an endoscopic naso-pancreatic stent-guided single-branch resection of the pancreas for branch-type multiple intraductal papillary mucinous adenomas, along with a gastric wall-covering method for the prevention of pancreatic leakage.
- Citation: Kuroki T, Tajima Y, Tsutsumi R, Tsuneoka N, Kitasato A, Adachi T, Kanematsu T. Endoscopic naso-pancreatic stent-guided single-branch resection of the pancreas for multiple intraductal papillary mucinous adenomas. World J Gastroenterol 2006; 12(44): 7203-7205
- URL: https://www.wjgnet.com/1007-9327/full/v12/i44/7203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i44.7203
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is characterized by dilatation of the main and/or branch pancreatic ducts, mucin-secretion, and intraductal papillary growth. IPMNs are classified into three types, a main pancreatic duct type, branch type, and mixed type. The branch type IPMN is more frequently located in the head of the pancreas, and shows less malignant potential[1,2]. Therefore, several new surgical procedures for minimal resection of the pancreas with pancreatic functional reserve have been proposed, including duodenum-preserving pancreatic head resection, segmental pancreatectomy and single-branch resection of the pancreas[3-5]. Single-branch resection is a minimally invasive surgical procedure for the resection of the pancreas because it requires no reconstruction for the main pancreatic duct or the common bile duct. In addition, single-branch resection should avoid injury to the main pancreatic duct which could cause a postoperative pancreatic fistula. Although several surgical techniques and devices have been advocated to avoid breaking the main pancreatic duct, the incidence of pancreatic fistula is still relatively high[6]. We report herein a case of successful single-branch resection for two IPMNs within the head of the pancreas under the guidance of intraoperative pancreatography by using an endoscopic naso-pancreatic drainage (ENPD) tube along with a gastric wall-covering technique for the prevention of pancreatic leakage.
A 59-year-old man was referred to our hospital for further evaluation of two cystic tumors in the head of the pancreas which were detected by routine ultrasonography (US). Upon admission, there were no abnormal findings in the laboratory data. The endocrine pancreatic function by a 75 g oral glucose tolerance test and the exocrine function by a pancreatic function diagnostic (PFD) test were within normal range. Abdominal US and computed tomography (CT) showed two multilobular cystic lesions, 15 mm and 40 mm in diameter, respectively, in the head of the pancreas. Endoscopic retrograde pancreatography (ERP) showed two cystic lesions communicating with the main pancreatic duct. Although mucous secretion was detected from the duodenal papilla, the main pancreatic duct showed no abnormality. Magnetic resonance cholangiopancreatography (MRCP) and ERP revealed a communicating branch to these two cystic lesions (Figure 1). No mural nodules were noted in the cysts. The cytology of the pancreatic juice was negative for malignant cells. K-ras point mutation at codon12 was detected in the pancreatic juice. Consequently, the cystic lesions were diagnosed as a branch-type intraductal papillary mucinous neoplasm (IPMN) and the patient was considered a candidate for surgery.
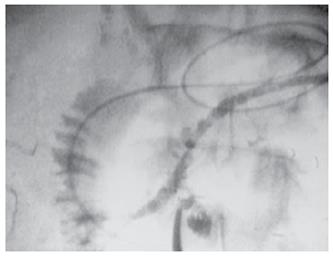
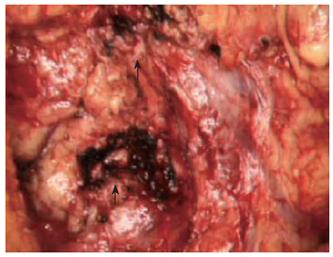
Under general anesthesia, we placed a 5F-size ENPD tube (Olympus, Tokyo Japan) for intraoperative pancreatography prior to laparotomy. The ENPD tube was inserted deep into the main pancreatic duct for approximately 10 cm through the orifice. After an upper abdominal midline incision, the gastrocolic ligament was divided and the pancreas exposed. The duodenum was mobilized to facilitate access to the head of the pancreas. The communicating branches between the cystic lesions and main pancreatic duct were carefully evaluated by using intraoperative US and pancreatography (Figure 2), and a single-branch resection for both cystic tumors was then performed. During the resectioning of the cystic tumors, the ENPD tube in the main pancreatic duct was clearly and easily detected by intraoperative US, and thus both the direction and position of the main pancreatic duct and the correlation between the cystic tumors and main pancreatic duct were correctly identified. The two cystic tumors were dissected along the border of the tumor and the surrounding normal pancreatic parenchyma, and then the confluences of the pancreatic branch ducts and main pancreatic duct were ligated using a 4-0 non-absorbable monofilament suture. Intraoperative frozen section histological examination of the dissected pancreatic ducts revealed a disease-free margin. After removal of the tumors, pancreatography through the ENPD tube was performed and revealed no injury to the main pancreatic duct or leakage from the transected pancreatic branch ducts. Because a mixture of indigocarmine and contrast material was used for the pancreatography, we were able to detect any minor pancreatic leakage as a blue spot at the cut surface of the pancreas and close the leaking points appropriately with 4-0 absorbable monofilament sutures. After pancreatic resection, the ENPD tube was used for the drainage of the pancreatic duct. It was not necessary to perform any reconstruction of the digestive tract (Figure 3). To prevent pancreatic leakage from the small pancreatic branches at the cut surface of the pancreas, the “gastric wall-covering method 7” was performed as follows: each cut surface after resection of the two cystic tumors was fixed to the posterior wall of the gastric body by suturing between the pancreatic parenchyma and the seromuscular layer of the stomach with 4-0 absorbable monofilament. The operating time was 225 min, and blood loss was 240 mL. Histological diagnosis was intraductal papillary mucinous adenoma of the pancreas. The ENPD tube was removed on the 14th postoperative day. The postoperative course was uneventful, and the patient was discharged home on the 25th postoperative day with no endocrine or exocrine pancreatic insufficiency.
The branch type of IPMN of the pancreas shows a more favorable prognosis than invasive ductal adenocarcinoma of the pancreas. In the benign or low-grade malignant IPMN, complete tumor resection is sufficient for a cure[1,2]. Therefore, several organ-preserving techniques, including duodenum-preserving pancreatic head resection, have been advocated in the surgical treatment for IPMN in the head of the pancreas[3]. The advantage of duodenum-preserving pancreatic head resection is the complete preservation of the duodenum, including the duodenal papilla sphincter function, compared with a pylorus-preserving pancreaticoduodenectomy. However, the duodenum-preserving pancreatic head resection requires a reconstruction of the main pancreatic duct and/or common bile duct. In addition, another challenge when performing the duodenum-preserving pancreatic head resection is preserving the blood supply to the duodenum. Therefore, single-branch resection of the pancreas for multiple IPMNs has been performed as an ultimate organ-preserving surgery[5]. In the present case, we developed single-branch resection of the pancreas using an ENPD tube for multiple branch-type IPMNs as an ideal pancreas-preserving surgery. The most important advantage of the single-branch resection is both the complete preservation of the main pancreatic duct and the complete removal of the lesions. The insertion of an ENPD tube was useful for identifying the pancreatic duct in order to prevent injury to the main pancreatic duct. The ENPD tube in the main pancreatic duct is easily detectable using intraoperative US, and it can demonstrate a repeated intraoperative pancreatography. The first benefit of intraoperative pancreatography using an ENPD tube is that the identification of the anatomical relationship between the cystic lesions and main pancreatic duct can be confirmed not only by a radiological image, but also by a direct palpation. In addition, we used a mixture of a dye and contrast medium for the intraoperative pancreatography, which allowed us to identify the pancreatic leakage directly as a visible blue point by detecting the dye leakage. Pancreatic leakage is the most frequent complication and is still responsible for most mortality after pancreatic surgery[6]. In particular, the incidence of pancreatic leakage following partial resection of the pancreas is high. In the present case, a novel surgical procedure, a “gastric wall-covering method,” was performed to prevent pancreatic leakage[7]. The concept of the gastric wall-covering method is that the opening small pancreatic ducts at the cut surface as a cause of pancreatic leakage were covered completely with the gastric wall. As shown in our patient, the multiple partial resections of the pancreas had a wide cut surface. Therefore, the gastric wall-covering method is useful for the prevention of pancreatic leakage from the opening of the small pancreatic branch ducts at the cut surface.
In conclusion, single-branch resection of the pancreas, without reconstruction of the pancreatobiliary tract, is the most ideal, least invasive pancreatic resection for a low-grade malignant neoplasm, including multiple IPMNs. ENPD tube-guided pancreatectomy is an improved technique that allows the surgeon to more accurately determine the precise location and correlation of tumors and pancreatic ducts.
| 1. | Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004;28:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Kimura W. IHPBA in Tokyo, 2002: surgical treatment of IPMT vs MCT: a Japanese experience. J Hepatobiliary Pancreat Surg. 2003;10:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Takada T, Yasuda H, Amano H, Yoshida M. A duodenum-preserving and bile duct-preserving total pancreatic head resection with associated pancreatic duct-to-duct anastomosis. J Gastrointest Surg. 2004;8:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Nakagohri T, Kenmochi T, Kainuma O, Tokoro Y, Kobayashi S, Asano T. Inferior head resection of the pancreas for intraductal papillary mucinous tumors. Am J Surg. 2000;179:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Sata N, Koizumi M, Tsukahara M, Yoshizawa K, Kurihara K, Nagai H. Single-branch resection of the pancreas. J Hepatobiliary Pancreat Surg. 2005;12:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kuroki T, Tajima Y, Kanematsu T. Surgical management for the prevention of pancreatic fistula following distal pancreatectomy. J Hepatobiliary Pancreat Surg. 2005;12:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kuroki T, Tajima Y, Tsutsumi R, Mishima T, Kitasato A, Adachi T, Kanematsu T. Inferior branch-preserving superior head resection of the pancreas with gastric wall-covering method for intraductal papillary mucinous adenoma. Am J Surg. 2006;191:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH