INTRODUCTION
Insulin resistance emerges as a very important host factor in patients with chronic hepatitis C, mainly because it has been related to steatosis development, fibrosis progression and non-response to peginterferon plus ribavirin. IR appears as a consequence of the inability of insulin to induce the effect on glucose metabolism and an abnormally large amount of insulin is secreted to achieve a biological response. The hyperinsulinemic state induces several abnormalities in the liver, endothelium, and kidneys, and is the main feature in the metabolic syndrome. Obesity and metabolic syndrome are frequently found in western countries. In Spain, the prevalence of obesity has been found in around 13% and more than half of the population showed increased body mass index. In chronic hepatitis C, overweight and obesity showed a distribution similar than healthy people. Although insulin resistance has been found to be strongly associated with body mass index, in some circumstances it depends on the virus C. Two types of insulin resistance could be defined in patients with chronic hepatitis C: “metabolic” insulin resistance and “viral” insulin resistance.
MATERIALS AND METHODS
Insulin, after binding with it’s receptor, induces the phosphorylation of receptor substrates in the liver and muscles and induces several steps toward the transactivation of GLUT-4 that increases glucose uptake by cells and the storage as glycogen and inhibits the net production of glucose by the liver, blocking glycogenolysis and neoglycogenesis. Moreover, insulin promotes lipids storage inhibiting lipolysis. When insulin is not able to induce glucose uptake, pancreatic beta-cells increase insulin production and the hyperinsulinemic state avoids hyperglycemia. Insulin secretion increases when insulin sensitivity decreases until a threshold in which insulin secretion did not induce improvement in insulin sensitivity, and diabetic state emerges. Several factors have been proposed as mediators in the islet adaptation: glucose, glucagon-like peptide 1, autonomic nervous system, and free fatty acids[1].
The best method to measure insulin sensitivity is the hyperinsulinemic euglycemic clamp. This method measures the quantity of glucose required to maintain normal glycemic levels during insulin infusion. Briefly, a perfusion of 60 mU/kg per minute of insulin was started and every 5 minutes glucose levels were measured. To avoid hypoglycaemia, glucose (20%) was infused to maintain levels between 90 mg/dL and 100 mg/dL. The whole-body glucose uptake (M value) depends on the glucose infused in the last 30 min of the test. Lower M value (lower glucose requirements) are associated with insulin resistance. However, the clamp method is expensive, labor-intensive and uncomfortable for practical use in clinical medicine. A simple mathematical model named Homeostasis Model for Assessment [HOMA = fasting insulin (mUI/mL) * fasting glucose (mmol/L)/22.5] has been proved useful in the measurement of insulin sensitivity in euglycemic patients. However, this measurement is not useful once the blood glucose level begins to increase because it lacks accuracy. Normal plasma insulin concentrations are not standardized in healthy controls. Thus, HOMA has not been standardized yet. In previous reports HOMA < 2 has been considered “completely” normal, and higher than 4 as pre-diabetic state.
INSULIN RESISTANCE IN CHRONIC HEPATITIS C
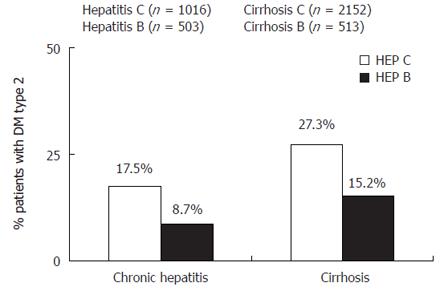
The mechanisms by which hepatitis C induces increased insulin resistance and the risk for development of diabetes has not been completely understood. Liver fibrosis progression has been considered for long-time responsible of the appearance of insulin resistance and type 2 diabetes in patients with chronic liver diseases. Taking together available data from several studies about the prevalence of type 2 diabetes mellitus in hepatitis C, we found that in cirrhotic patients (n = 2152 cases) the prevalence of type 2 diabetes was 27.3%, higher than the 17.5% in non-cirrhotic hepatitis C patients (n = 1016 cases). Hyperinsulinemia in liver cirrhosis has been reported to be due to diminished hepatic insulin extraction by liver dysfunction and not to pancreatic hypersecretion. C-peptide and insulin are secreted in equimolar quantities. More than 50% of insulin is degraded in the liver at first pass, whereas C-peptide is degraded in the kidneys[2]. Simultaneous measurements of C-peptide and insulin revealed that both insulin resistance and insulin secretion contribute to glucose intolerance in patients with chronic hepatitis C[3]. In spite of insulin resistance correlated with liver fibrosis, it has been found higher in patients with chronic hepatitis C and mild fibrosis (F0-F1) than in healthy controls matched by age, sex, visceral obesity and body mass index[4]. In a cohort of patients with chronic hepatitis C and chronic hepatitis B matched by age, sex, body mass index and fibrosis stage HOMA index was found statistically higher in hepatitis C[5], this result confirms previous reports showing higher insulin resistance in chronic hepatitis C than in others hepatopathies[6]. Thus, hepatitis C virus induces insulin resistance independently of body mass index or fibrosis stage. Moreover, in a transgenic animal model, HCV core protein has been found able to induce insulin resistance, steatosis and type 2 diabetes mellitus. The main mechanism seems to be the over production of TNF. This cytokine phosphorylates serine residues of insulin-receptor substrates 1 and 2 and enhances the production of suppressor of cytokines (SOC3). The SOC-3 substance inhibits the phosphorylation of Akt and phosphatidyl inositol 3 kinase (PI3K). All these impairments in the intracellular signaling of insulin could block the transactivation of GLUT-4, avoiding the uptake of glucose by cells. Moreover, in transgenic animal models unable to express SOC-3, in spite of core HCV protein expression, insulin resistance does not develop. Indeed, in transgenic mice, TNF correlates with hyperinsulinemic state and the blockade of TNF production by anti-TNF drugs as infliximab also avoid insulin resistance appearance. Therefore, mechanisms inducing insulin resistance by hepatitis C virus include: TNF production, serine phosphorylation of IRS, and over expression of SOCs. Furthermore, over production of TNF in patients with chronic hepatitis C has frequently been seen[7], and correlated with higher fibrosis progression and impaired antiviral response to interferon alpha. Besides, in humans the risk for the development of insulin resistance and further appearance of DM is greater in patients with higher TNF production.
The development of type 2 diabetes mellitus depends on environmental, genetic and diet-related factors. Type 2 diabetes mellitus is more often seen in patients with chronic liver diseases than in general population[8]. Data supporting an association between diabetes mellitus and hepatitis C include: (1) Cross-sectional studies that found increased prevalence of diabetes mellitus in patients with chronic hepatitis C, or higher prevalence of hepatitis C in patients with diabetes mellitus. In a recent meta-analysis taking together available data about the prevalence of DM in patients with chronic liver diseases, the prevalence was higher in chronic hepatitis C than chronic hepatitis B. In non-cirrhotic chronic hepatitis C, DM was found in 17.5% and in chronic hepatitis B in 8.7% while in cirrhotics the prevalence was 27.3% in hepatitis C versus 15.2% in cirrhotics B (Figure 1). Besides, anti-HCV was more often detected in patients with diabetes mellitus (4.9%) than in the general population (0.77%)[9]. (2) case-cohort studies to analyze the development of diabetes mellitus during follow-up in patients with hepatitis C against uninfected patients, including follow-up after orthotopic liver transplantation. In a cross-sectional survey including 9841 persons, Mehta et al found that HCV-positive persons who were older than 40 years had an increased risk for type 2 diabetes mellitus higher than 3 times compared with persons without HCV-infection, while no difference was seen in HBV infection[10]. In a cohort of 1084 patients followed-up for 9 years 548 cases developed diabetes mellitus. The presence of hepatitis C was associated with a greater development of diabetes, but solely in high-risk diabetes patients[11]. Besides, in a cohort of 2327 cases, hepatitis C infection increased three times the rate of diabetes mellitus appearance during follow-up in patients aged between 35 and 49 years[12]. Thus, HCV infection could promote type 2 diabetes in high-risk populations.
Figure 1 Prevalence of type 2 diabetes mellitus in patients with chronic hepatitis C and chronic hepatitis B.
Recently, in a Spanish study including 525 chronic hepatitis C patients treated with peginterferon plus ribavirin were followed-up after treatment and the incidence of altered baseline glucose and the appearance of diabetes type 2 was greater in non-responders than in sustained responders even after multivariate analysis including confounding variables as previous diabetes type 2 in relatives, older than 40 years and male sex. Thus, hepatitis C virus clearance induces a decrease in insulin resistance index during a short-time follow-up and decrease the incidence of diabetes type 2 in long-term follow-up[13].
Hepatocyte steatosis is a common feature found in the liver of patients with chronic hepatitis C. The mechanisms implied in steatosis appearance seem to be genotype-dependent. In patients infected by genotype 3, steatosis emerges as a cytopathic effect of the virus while in genotype 1 steatosis seems to be an expression of the metabolic syndrome. In genotype 3, steatosis degree correlated with liver and serum HCV load[14]. In genotype 1, steatosis depends on leptin levels and insulin resistance. Recently, insulin resistance has been found implied in the development of steatosis in 331 non-diabetic genotype 1 patients. Insulin resistance together with gender and gammaglutamyl-transpeptidase (a surrogate marker of TNF levels) were independently associated with moderate/severe steatosis in this cohort[15]. Insulin resistance is the main pathogenic factor in the development of steatosis in chronic hepatitis C, both viral insulin resistance and metabolic insulin resistance could be implied in the development of steatosis.
The main deleterious effect of insulin resistance in chronic hepatitis C is the ability to promote fibrosis progression. High serum glucose levels have been found associated with an increased rate of fibrosis progression, greater even than overweight[16]. Mean HOMA index increases with the stage of fibrosis[17] and could help to differentiate stages of fibrosis. Recently, Sud et al[18], proposed an index to predict fibrosis containing age, cholesterol, gammaglutamyl transpeptidase and alcohol consumption together with HOMA. The mechanisms by which insulin resistance promotes fibrosis progression include: (a) steatosis, (b) hyperleptinemia, (c) increased TNF production, and (d) impaired expression of PPARγ receptors. (a) hepatocyte steatosis induces fibrosis progression. In a cohort of patients with chronic hepatitis C, the presence of steatosis in the first biopsy was associated with higher fibrosis progression rate, irrespective of genotype[19]. Besides, steatosis is more often seen in patients with advanced fibrosis[20]. (b) Hyperleptinemia has been usually found in patients with insulin resistance, also hepatic stellate cell showed specific leptin receptors, thus leptin could play a role in the activation of HSC and fibrosis progression[21,22]. (c) TNF production is enhanced in hepatitis C and has been implicated in the development of insulin resistance, also, TNF levels were related to fibrosis progression, owing to the ability to activate HSC and promote collagen deposits. Moreover, TNF could inhibit PPARs activity[23]. (d) In patients with hepatitis C an impaired expression of PPARγ receptors has been found[24]. PPARs agonist inhibits inflammation and fibrosis progression by blocking the activation of redox-sensitive transcription factor NFκB and TGFβ1[25].
In HIV-infected patients insulin resistance depends on host, virus and drugs-related factors. Adverse metabolic effects have been found in all antiretroviral drug classes. Protease inhibitors (PI) and nucleoside reverse transcriptase inhibitor (NRTI) therapy induces insulin resistance during treatment[26]. Insulin resistance has been found increased in HIV+/HCV+ co-infected patients. Factors implied in the development of insulin resistance in HIV infected patients include: HCV infection, PI and/or NRTI based-therapy, age, HIV and cytokine dysregulation induced by chronic infection and genetic predisposition[27]. Moreover, the development of type 2 diabetes mellitus was twice in co-infected patients versus HIV non-HCV infected[28]. Duong et al[29] reported a higher HOMA in co-infected patients than in HIV-infected cases without hepatitis C. However, in a large study no differences were seen between co-infected or not co-infected patients. In a recent study, including 127 co-infected patients that underwent antiviral therapy (peginterferon plus ribavirin) and 85 hepatitis C, insulin resistance index was higher in co-infected patients, in spite of lower body mass index and lower baseline glucose levels. Besides, in a genotype-dependent manner, insulin resistance was strongly associated with steatosis development and impaired the sustained response rate in genotype 1 co-infected patients. No association between insulin resistance and steatosis or sustained response was seen in genotype 2 or 3 coinfected patients[30]. Lastly, history of diabetes in relatives, increased body mass index and HCV-infection have been found as independent variables associated with diabetes development in HIV patients, supporting the hypothesis that HCV infection promotes diabetes in high-risk patients.
INSULIN RESISTANCE AND RESPONSE TO PEGINTERFERON PLUS RIBAVIRIN
In healthy volunteers, after the first injection of interferon alpha insulin resistance could be detected[31]. Besides in patients with chronic hepatitis C, interferon alpha induces insulin resistance in the first two weeks, mainly owing to a decrease of hepatic glucose uptake[32]. This effect has been related to the pro-inflammatory repertoire of cytokines induced by interferon. However it’s a transitory effect because insulin resistance was not found at month three of therapy[33] or at the end of therapy[34]. In patients with chronic hepatitis C receiving peginterferon plus ribavirin insulin resistance measured by HOMA, decreased in patient with HCV RNA clearance at mo 6, but not in non-responders. At the end of follow-up sustained responders showed a significantly lower HOMA in comparison with baseline insulin resistance index. However, in relapser patients, the HOMA index increased and the levels at the end of follow-up were not different from the baseline. These data support a connection between HCV replication and insulin resistance, and HOMA decreased when the virus was eradicated. Besides, the incidence of diabetes type 2 is different in cured patients than in non-responders, supporting a better control of insulin resistance after hepatitis C virus clearance[13].
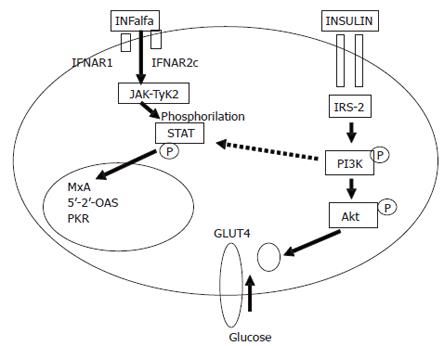
Genotype and viral load have been found as the most important viral factor influencing peginterferon plus ribavirin response. Host factors include genes as HLA, weight, body mass index, hepatocyte steatosis, age, Afro-American ethnicity and fibrosis. Several genes and polymorphisms as HLA-B44 has been found related to the possibility of curing the disease[35]. On the other hand, features of metabolic syndrome as overweight, steatosis and fibrosis have been reported as independently associated with response. The influence of steatosis in the chance of curing hepatitis C has been controversial for long time. Steatosis impairs SVR rate in patients with genotype 1, but not in genotype 3. Thus, the possibility of finding steatosis as independent variable depends on the balance between genotypes included in the cohort. In fact, in a multivariate analyses including steatosis together with fibrosis, body mass index, genotype and insulin resistance, either steatosis or body mass index were not found independently associated with response but insulin resistance, genotype and fibrosis[32]. In genotype 1, sustained response rate was 32% in patients with insulin resistance (HOMA > 2) against 60% in patients with HOMA lower than 2[36]. However, in a recent cohort including 331 non-diabetic genotype 1 chronic hepatitis C patients, steatosis but not insulin resistance was found independently associated with response. Steatosis and insulin resistance showed a great colineality in genotype 1 patients and could be the expression of the same mechanisms. Thus, measure insulin resistance seems to be easier and comfortable than study steatosis in a liver biopsy. Besides, in 52 patients from UK also treated with peginterferon plus ribavirin, HOMA index was significantly higher in non-responders than patients with sustained response[37]. Thus, insulin resistance emerges as the most important host factors in the prediction of response in non-diabetic patients treated with the best available option peginterferon plus ribavirin. Interestingly, insulin resistance has been found as a common denominator to the majority of features associated with difficult-to-treat patients: patients with cirrhosis, obesity, co-infected by HIV and Afro-American. Unresolved question is whether insulin resistance is a marker of very difficult-to-cure or a pathogenic mechanism able to block antiviral activity of the interferon. Peginterferons induce their antiviral activity via extracellular receptor binding. The interferon alpha signalling pathway involves the activation of Janus kinase (Jak1) and tyrosine kinase (Tyk2), initiated by the binding of peginterferon alpha-2 to the interferon heterodimeric receptor complex (IFNAR1/IFNAR2), which leads to activation of their downstream substrates, signal transducers and activators of transcription (STAT 1 and STAT2). Activated STAT then assemble as a multimeric complex and translocate into the nucleus where they bind to interferon alpha-2-stimulated response elements in the promoters of interferon alpha-2-stimulated genes[38]. Recently, in a replicon model using Huh-7 cells transfected by full length HCVRNA, interferon alpha blocks HCV replication. However, when insulin (at doses of 128 μU/mL, similar that seen in the hyperinsulinemic state in patients with metabolic syndrome) was added to interferon, the ability to block HCV replication disappeared, and the PKR synthesis was abolished[39]. In this experiment, a blockage of PI3K by LY294 002 avoided the interference of insulin, supporting that interferon resistance induced by insulin is mediated by PI3K (Figure 2). Moreover, in genotype 1 patients obesity has been found able to increase intrahepatic synthesis of SOC-3, inhibiting the interferon signalling and promoting interferon resistance[40]. Thus, hyperinsulinemia and obesity induce interferon resistance blocking intracellular signalling.
Figure 2 Interaction between insulin and peginterferon alpha signalling pathway.
The phosphatidil-inositol-3-kinase (PI3K) activated by insulin seems to be responsible for the block of STAT 1 translocation avoiding antiviral effect of interferon. Dotted lines represent inhibition, continuous lines represent activation.
CONCLUSIONS
In summary, hepatitis C promotes insulin resistance and insulin resistance induces interferon resistance, steatosis and fibrosis progression in a genotype-dependent manner. In genotype 1 insulin resistance decreases sustained response rate, and increase the risk for the development of steatosis and fibrosis progression, in both, coinfected HCV+/HIV+ and in hepatitis C. However, the impact of insulin resistance in non-1 genotype seems not achieve enough importance to impair sustained response, probably due to the high sensitivity to peginterferon. The treatment of insulin resistance, decreasing hyperinsulinemia, could improve sustained response rate in genotype 1 patients with chronic hepatitis C when treated with peginterferon plus ribavirin. A multicenter Spanish trial (TRIC-1) is ongoing to analyze the effect of triple therapy with peginterferon alfa-2a plus ribavirin and metformin versus standard therapy and placebo in patients with chronic hepatitis C infected by genotype 1 and showing insulin resistance.
ACKNOWLEDGMENTS
The author thanks Drs. J Salmerón (Hospital San Cecilio, Granada), R Andrade (Hospital Virgen de la Victoria, Málaga), C Fernández-Rodríguez (Hospital Fundación Alcorcón, Madrid), M Diago (Hospital General de Valencia, Valencia), C Tural (Hospital Germans Trias i Pujol, Badalona), MC Martínez-Sierra (Hospital Puerta del Mar, Cádiz), R Sola (Hospital del Mar, Barcelona), R Planas (Hospital Germans Trias i Pujol, Badalona), B Clotet (Hospital Germans Trias i Pujol, Badalona) for the works done together in insulin resistance in hepatitis C and coinfected HCV-HIV patients. We also like to thank Drs F Recio, J Castillo, M Cruz, I Camacho and MM Viloria (Hospital Universitario de Valme, Sevilla).
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu WF