Published online Nov 21, 2006. doi: 10.3748/wjg.v12.i43.6973
Revised: October 2, 2006
Accepted: October 11, 2006
Published online: November 21, 2006
AIM: To compare gemcitabine-based combination therapy and gemcitabine (GEM) alone in patients with advanced pancreatic cancer (APCa) through meta-analysis.
METHODS: MEDLINE and EMBASE searches were supplemented by information from trial registers of randomized controlled trials (RCTs) for GEM-based combination therapy and GEM alone for APCa. A quantitative meta-analysis was carried out by two reviewers based on the inclusion criteria from all available RCTs. The meta-analysis involved overall survival (OS), objective remission rate (ORR), clinical benefit rate (CBR), time to progress/progress free survival (TTP/PFS) and toxicity.
RESULTS: The meta-analysis included 22 RCTs. There was significant improvement in the GEM combination group with regard to the 6-mo survival rate (RD = 0.04, 95% CI 0.01-0.06, P = 0.008), 1-year survival rate (RD = 0.03, 95% CI 0.01-0.05, P = 0.01), ORR (RD = 0.04, 95% CI 0.01-0.07, P = 0.02), CBR (RD = 0.10, 95% CI 0.02-0.17, P = 0.01) and 6-mo TTP/PFS (RD = 0.07, 95% CI 0.04-0.10, P < 0.00001). However, the Grade 3-4 toxicity set by WHO was higher for the GEM combination group for neutropenia (RD = 0.05, 95% CI 0.01-0.10, P = 0.02), thrombocytopenia (RD = 0.05, 95% CI 0.02-0.08, P = 0.002) and vomiting/nausea (RD = 0.03, 95% CI 0.00-0.05, P = 0.02).
CONCLUSION: GEM-based combination therapy may improve the overall survival and palliation in optimal patients with APCa as compared with GEM alone.
- Citation: Xie DR, Liang HL, Wang Y, Guo SS, Yang Q. Meta-analysis on inoperable pancreatic cancer: A comparison between gemcitabine-based combination therapy and gemcitabine alone. World J Gastroenterol 2006; 12(43): 6973-6981
- URL: https://www.wjgnet.com/1007-9327/full/v12/i43/6973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i43.6973
Gemcitabine (GEM) monotherapy currently is considered as a standard treatment for patients with advanced pancreatic cancer (APCa). However, patients treated with GEM alone have poor prognoses, and their overall median survival (OS) was only 5.65 mo[1]. Attempts have been made to increase the objective remission rate (ORR) and survival of APCa patients, in particular, by exploring the effects of the combined GEM with other drugs. In many phase II studies, GEM combinations have improved ORR and OS. Based on these results, many prospective, randomized phase III trials comparing GEM used in combination and alone have been carried out. But these trials had different results and the population enrolled is small. Therefore, the NCCN guidelines (National Comprehensive Cancer Network, v.2.2006) indicate that GEM-based combination therapy may be an optimal treatment for APCa patients with a good performance status, including GEM + cisplatin (DDP), GEM + oxaliplatin, GEM + capecitabine, GEM + erlotinib and so on. But these guidelines were based on low level evidence including clinical experience (category 2A). The role of GEM-based combination therapy for the treatment of APCa is still unclear. We therefore, conducted a systematic review and quantitative meta-analysis to evaluate the available evidence from the relevant randomized trials.
We carried out a comprehensive search of literature with MEDLINE (1966-2006), EMBASE (1966-2006), CBMdisc (1981-2006), ASCO Abstracts (1995-2005) and EBM Reviews (Cochrane Database of Systematic Reviews 1st Quarter, 2006) ACP Journal Club (1991-2006), (Database of Abstracts of Reviews of Effects 1st Quarter 2006), Cochrane Central Register of Controlled Trials (1st Quarter, 2006), using the terms: ’pancreas’, ’pancreatic cancer’, ’pancreatic carcinoma, ’pancreatic adenocarcinoma’, ’pancreatic neoplasms’, ’gemzar’, ’gemcitabine’ (no limit to language). Date of last search: April 26, 2006.
Study design: Trials should be prospective, properly randomized and well designed, which were matched for age, stage, performance status, etc.
Study population: Patients with APCa, as well as those with locally advanced, or metastatic disease, were included in the study. Patients eligible for the study were required to have histologically or cytologically proved pancreatic cancer. Furthermore, they should have a baseline Karnofsky performance status of ≥ 50% (or Eastern Cooperative Oncology Group performance status < 2) and adequate hematological, renal, cardiac and hepatic function. Patients with estimated life expectancy of at least 12 wk, should have received no chemotherapy, radiotherapy and other antitumor therapy in the 6 mo prior to the study entry.
Intervention: The treatment group received GEM-based combination therapy, and the control group received GEM alone.
Outcome: The primary outcome measurement was OS, followed by ORR and toxicity. The follow-up rate should be above 95%.
Data collection and analysis: Two reviewers assessed the abstracts identified from the defined sources. Both reviewers independently selected trials for inclusion according to prior agreement regarding the study population and the intervention. If one of the reviewers concluded an abstract to be eligible, the full text of article was retrieved and reviewed in detail by both reviewers. Missing data from the primary study reports was requested from the investigators. If the same trial appeared on different publications, the final data of the trial was chosen. Methodologic quality of the trials was assessed using a validated scale (range, 0 to 5) applied to items that influence the intervention efficacy. It was reported by Jadad et al[2] that the scale consisted of items pertinent to randomization, masking, dropouts, and withdrawals. The following information was obtained from each trial: year of publication, number of patients, performance status, chemotherapy regimen, objective response rate, overall survival, progress free survival, clinical benefit, toxicity, etc. For response assessment, we used trials that included patients with measurable or assessable diseases, and that were analyzed mainly with WHO’s criteria. Toxicity profiles were reported according to the WHO’s criteria.
The primary end point was a 6-mo survival rate after randomization. The other end points were 1-year survival rate, ORR, CBR, 6-mo TTP/PFS rate and adverse effects. All variables were defined as dichotomous data (e.g., 6-mo survival rate used variables as follows: the alive or dead at 6 mo after randomization). We standardized the therapeutic results by obtaining the risk difference (RD) between the GEM combination group and the GEM alone group. Publication bias was examined using a funnel plot[3]. We looked for heterogeneity among the trials based on Cochran’s χ2 test. All analyses were performed strictly with RevMan software (version 8.2, Cochrane). P value less than 0.05 was considered as significant in difference.
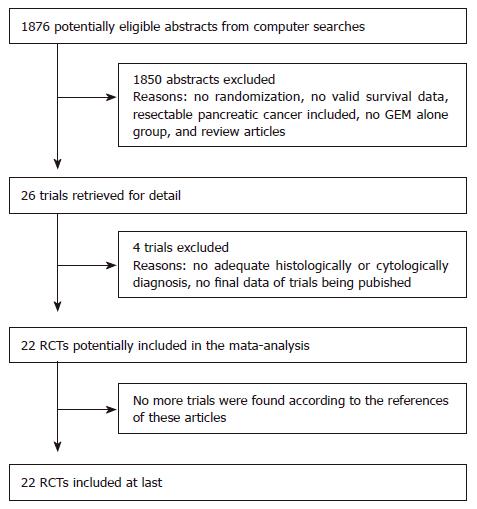
The flow chart of our study is shown in Figure 1. Because the trial reported by Degen et al[4] involved some patients diagnosed by imageology, we excluded this trial from our analysis. Of the 26 trials, three reported by Ohkawa et al[5], Richards et al[6], and Shapiro et al[7], were excluded because of no final data. Both reviewers finally agreed to include 22 RCTs involving 5473 APCa patients in the meta-analysis.
These prospective randomized controlled studies are summarized in Table 1. All selected trials for inclusion strictly according to prior selection criteria, were prospective, randomized and well designed, and the clinical characteristics were matched for age, stage, performance status, and so on. All studies reviewed were considered high in quality, for they achieved a score of 3 or higher in the assessment scale of Jadad’s study design. Patients eligible for these studies had histologically or cytologically proved pancreatic cancer, with same baseline data and without evidence of selection bias. Of the 22 trials, seven were randomized phase II trials, and the others were randomized phase III trials. The 6-mo survival rate was extracted from each of the 22 trials, and objective remission rates were recorded in most of the trials. Only a few trials provided CBR, PFS, TTP and TTF (time of treatment failure).
| Studies | Intervention | Patients | OS (d) | 6-mo survival (%) | 1-yr survival (%) | 6-mo TTP/PFS/TTF rate (%) | ORR (CR + PR) % | CBR | Jadad score |
| Scheithauer 2003[8] | Gem | 42 | 246 | 59.4 | 37.2 | 24.6 (PFS) | 6/42 | 10/30 | 3 |
| Gem + Capecitabine | 41 | 285 | 67.7 | 31.8 | 36.9 | 7/41 | 15/31 | ||
| Colucci 2002[9] | Gem | 54 | 140 | 31.5 | 11 | 18 (TTP) | 5/48 | 21/43 | 3 |
| Gem + DDP | 53 | 210 | 47 | 11.3 | 28 | 14/45 | 20/38 | ||
| Wang XY 2002[10] | Gem | 20 | - | 81.3 | 31.3 | - | 1/16 | 14/16 | 3 |
| Gem + DDP | 22 | - | 61.6 | 11.1 | - | 2/18 | 14/20 | ||
| Gansauge 2002[11] | Gem | 28 | 144 | 32 | 11 | - | 1/28 | - | 3 |
| Gem + NSC-631570 | 28 | 279 | 64 | 29 | - | 6/28 | - | ||
| Berlin 2002[12] | Gem | 162 | 162 | 42 | 15.5 | 32/160 (PFS) | 9/162 | - | 3 |
| Gem + 5-FU | 160 | 201 | 55 | 21.9 | 41/158 | 11/160 | - | ||
| Bramhall 2002[13] | Gem + placebo | 119 | 164 | 43 | 17 | 23 (TTF) | 14/88 | - | 5 |
| Gem + marimastat | 120 | 165.5 | 47 | 18 | 29 | 11/97 | - | ||
| Cutsem 2004[14] | Gem + placebo | 347 | 182 | 49 | 24 | - | 28/347 | - | 5 |
| 341 | 193 | 53 | 27 | - | 20/341 | - | |||
| Louvet 2005[15] | Gem | 156 | 213 | 60.4 | 27.8 | 27.4 (PFS) | 27/156 | 26.9 | 3 |
| Gem1 + Oxaliplatin | 157 | 270 | 68 | 34.7 | 43 | 42/157 | 38.2 | ||
| Reilly 2004[16] | Gem | 174 | 186 | 51 | 21 | 27 (TTP) | 9/127 | - | 3 |
| Gem + DX-8951f | 175 | 201 | 54 | 23 | 39 | 12/147 | - | ||
| Richards 2004[17] | Gem | 282 | 189 | 50.9 | 20.1 | 27.6 (PFS) | 20/220 | - | 3 |
| Gem + Pemetrexed | 283 | 186 | 50.9 | 21.4 | 32.1 | 42/230 | - | ||
| Li CP 2004[18] | Gem | 25 | 138 | 20.3 | 13.6 | 11.8 (TTP) | 3/25 | 9/25 | 3 |
| Gem + DDP | 21 | 168 | 31.1 | 6.3 | 11.8 | 2/21 | 6/21 | ||
| Reni 2004[19] | Gem | 47 | - | 63.9 | 21.3 | 12.9 (PFS) | 4/47 | 5/20 | 3 |
| Gem + 5-FU + DDP + EPI | 52 | - | 64.6 | 38.5 | 37.4 | 20/52 | 15/23 | ||
| Viret 2004[20] | Gem | 41 | 201 | 58.3 | 25.1 | 10 (TTF) | 2/41 | - | 3 |
| Gem + DDP | 42 | 241 | 55.5 | 32.4 | 14 | 3/42 | - | ||
| Rocha Lima 2004[21] | Gem | 180 | 198 | 52.9 | 22 | 21.9 (TTP) | 8/180 | - | 3 |
| Gem + Irinotecan | 180 | 189 | 50.7 | 21 | 30.6 | 29/180 | - | ||
| Costanzo 2001[22] | Gem | 49 | 217 | 59 | 14.5 | - | 4/48 | - | 3 |
| Gem + 5-FU | 44 | 210 | 59 | 23.3 | - | 5/43 | - | ||
| Heinemann 2003[23] | Gem | 97 | 180 | 48.6 | 22.5 | 25.6 (TTP) | 8/93 | - | 3 |
| Gem + DDP | 95 | 228 | 59.4 | 27.5 | 39.3 | 10/92 | - | ||
| Kulke 2004[24] | Gem2 | 45 | - | 24/45 | - | - | - | - | 3 |
| Gem + DDP | 45 | - | 23/45 | - | - | - | - | ||
| Gem + Docetaxel | 49 | - | 22/49 | - | - | - | - | ||
| Gem + Irinotecan | 44 | - | 21/44 | - | - | - | - | ||
| Richards 2002[25] | Gem + Placebo | 88 | 213 | 62.9 | 20.4 | 25.9 (TTF) | 5/63 | - | 5 |
| Gem + CI-994 | 86 | 191 | 60.8 | 18.5 | 16.7 | 1/61 | - | ||
| Moore 2005[26] | Gem + Erlotinib | 285 | 191 | 58 | 24 | 32 (PFS) | 23/268 | - | 5 |
| Gem + placebo | 284 | 177 | 49 | 17 | 25 | 21/262 | - | ||
| Stathopoulos 2005[27] | Gem | 70 | 195 | 50 | 21.82 | - | 7/70 | - | 3 |
| Gem + Irinotecan | 60 | 192 | 60 | 24.29 | - | 9/60 | - | ||
| Riess 2005[28] | Gem | 236 | 186 | 53 | 20 | 30 (TTP) | 17/236 | - | 3 |
| Gem + 5-FU/CF | 230 | 175.5 | 49 | 20 | 29 | 11/230 | - | ||
| Herrmann 2005[29] | Gem | 157 | 219 | 62 | 27 | 42 (PFS) | 12/1523 | - | 3 |
| Gem + Capecitabine | 159 | 252 | 60 | 31 | 42 | 15/148 | - |
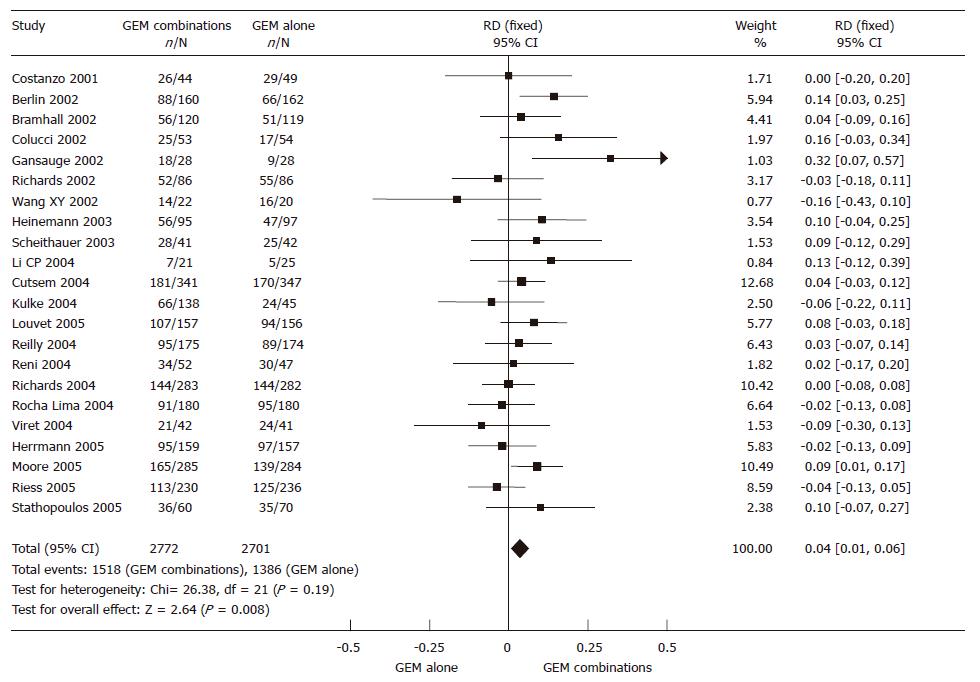
The 5473 randomized patients from 22 RCTs, 2772 in the GEM combination group and 2701 in the GEM alone group, were included in the meta-analysis. The result of the test for heterogeneity of the therapeutic effect was not significant (P = 0.19). Therefore, we selected a fixed effect model. There was a significant improvement in 4% of the GEM combination group in 6-mo survival rate (95% CI 0.01-0.06, P = 0.008). The results of the meta-analysis in 6-mo survival rate are presented in Figure 2.
With the same technique, 5292 patients from 21 RCTs were analyzed. In the GEM combination group, a 3% improvement was made in 1-year survival rate as compared with the GEM alone group, and this difference being statistically significant (95% CI 0.01-0.05, P = 0.01).
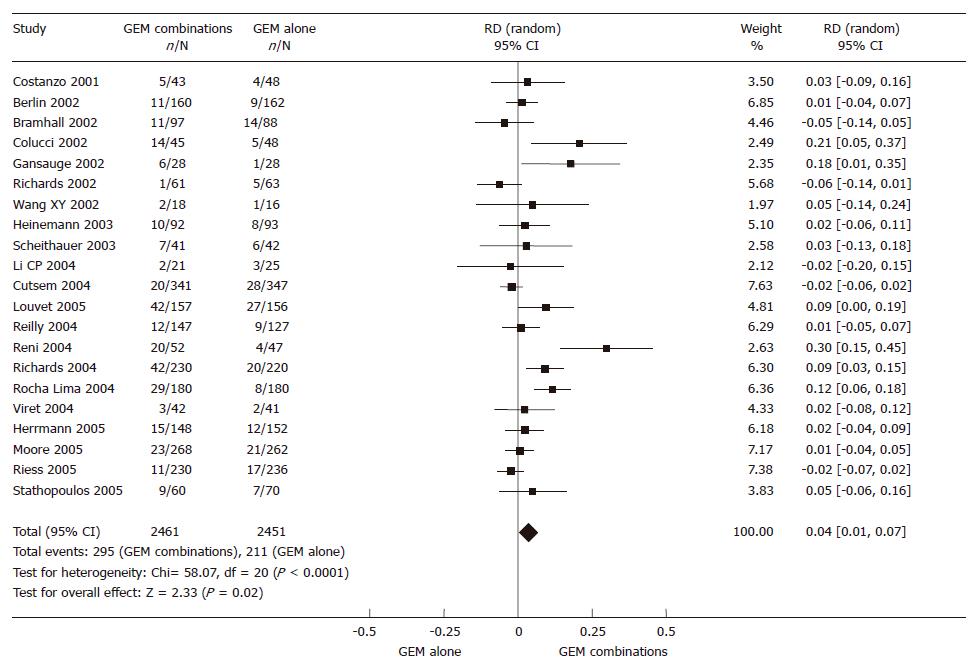
The 4912 randomized patients from 21 RCTs, 2461 in the GEM combination group and 2451 in the GEM alone group, were included in the meta-analysis. The result of the test for heterogeneity of the therapeutic effect was significant (P < 0.0001). A random effect model was adopted. There was a significant improvement in 4% of the GEM combination group in ORR (95% CI 0.01-0.07, P = 0.02). The outcome of the meta-analysis in ORR is presented in Figure 3.
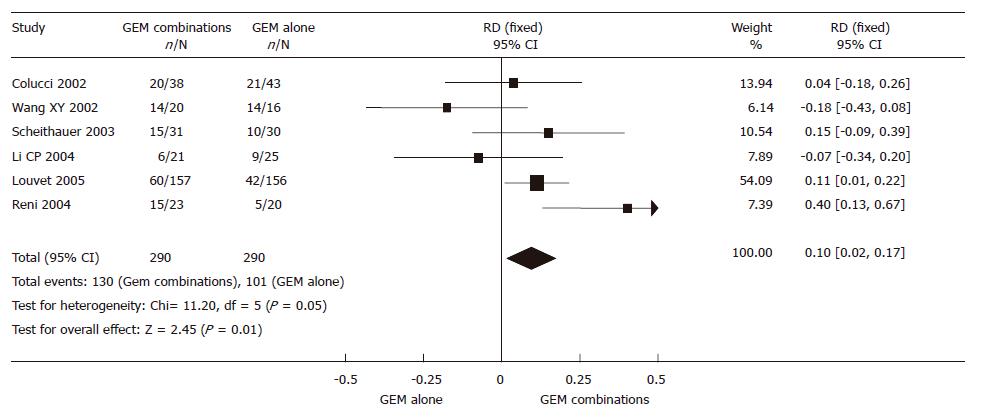
The 580 randomized patients from 6 RCTs, 290 in the GEM combination group and 290 in the GEM alone group, were included in the meta-analysis. The result of the test for heterogeneity of the therapeutic effect was not significant (P = 0.05).
A fixed effect model was used. There was a significant improvement in 10% of the GEM combination group in CBR (95% CI 0.02-0.17, P = 0.01). The outcome of the meta-analysis in CBR is shown in Figure 4.
TTP/PFS was defined as the period from randomization to documented disease progression for TTP or to disease progression or death for PFS. In almost all of the trials, patients recruited with good performance status died of disease progression, so TTP was very close to PFS. Therefore, we can analyze TTP and PFS together.
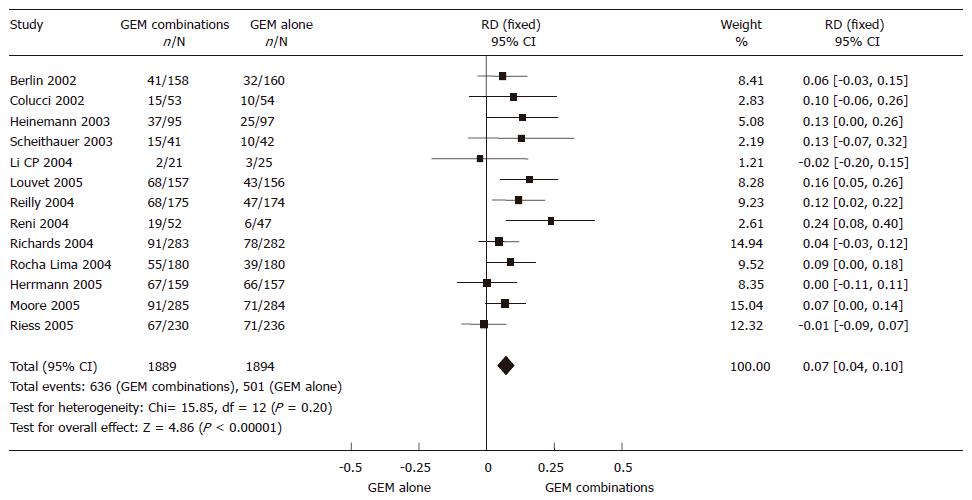
The 3783 randomized patients from 13 RCTs, 1889 in the GEM combination group and 1894 in the GEM alone group, were included in the meta-analysis. The result of the test for heterogeneity of the therapeutic effect was not significant (P = 0.20). A fixed effect model was used. Significant improvement was found in 7% of GEM combination group in 6-mo TTP/PFS rate (95% CI 0.04-0.10, P < 0.00001). The meta-analysis in TTP/PFS is presented in Figure 5.
Toxic effects of 21 RCTs are summarized in Table 2 (only Grade 3-4 toxic effects were recorded). Main toxic effects were analyzed. Grade 3-4 toxicity was higher in GEM combination group for neutropenia (RD = 5%, 95% CI 0.01-0.10, P = 0.02), thrombocytopenia (RD = 5%, 95% CI 0.02-0.08, P = 0.002) and vomiting/nausea (RD = 3%, 95% CI 0.00-0.05, P = 0.02), all reached significant difference.
| Studies | Intervention | Neutrophils | Platelets | Anemia | Infection | Nausea/vomit | Mucositis | Diarrhea |
| Scheithauer2003[8] | Gem | 3/39 | 1/39 | 0 | 0 | 0 | 0 | 0 |
| Gem + Capecitabine | 4/40 | 0 | 2/40 | 0 | 0 | 0 | 2/40 | |
| Colucci 2002[9] | Gem | 5/53 | 1/53 | 2/53 | - | 1/53 | 1/53 | 0 |
| Gem + DDP | 9/51 | 1/51 | 3/51 | - | 1/51 | 0 | 2/51 | |
| Wang XY 2002[10] | Gem | 5/19 | 7/19 | 2/19 | - | 0 | - | - |
| Gem + DDP | 7/21 | 8/21 | 9/21 | - | 2/21 | - | - | |
| Gansauge2002[11] | Gem | - | - | - | - | 3/28 | - | 1/28 |
| Gem + NSC-631570 | - | - | - | - | 1/18 | - | 0 | |
| Berlin 2002[12] | Gem | 8/158 | 17/158 | 16/158 | - | 30/158 | 3/158 | /158 |
| Gem + 5-FU | 11/158 | 30/158 | 16/158 | - | 29/158 | 2/158 | /158 | |
| Bramhall 2002[13] | Gem + placebo | 9/119 | - | 7/119 | 12/119 | 16/119 | - | - |
| Gem + marimastat | 3/120 | - | 3/120 | 11/120 | 13/120 | - | - | |
| Cutsem 2004[14] | Gem + placebo | 103/342 | 41/342 | 55/342 | - | 58/342 | - | 10/342 |
| Gem + R115777 | 132/331 | 50/331 | 66/331 | - | 46/331 | - | 13/331 | |
| Louvet 2005[15] | Gem | 2/156 | 5/156 | 16/156 | - | 12/156 | - | 2/156 |
| Gem + Oxaliplatin | 2/157 | 22/157 | 10/157 | - | 30/157 | - | 9/157 | |
| Reilly 2004[16] | Gem | 24/157 | 7/157 | 13/157 | - | 17/157 | - | 2/157 |
| Gem + DX-8951f | 50/168 | 28/168 | 11/168 | - | 33/168 | - | 6/168 | |
| Richards 2004[17] | Gem | 35/273 | 17/273 | 8/273 | - | 18/273 | - | 2/273 |
| Gem + Pemetrexed | 123/273 | 49/273 | 38/273 | - | 18/273 | - | 8/273 | |
| Li CP 2004[18] | Gem | 2/25 | 1/25 | 2/25 | - | - | - | - |
| Gem + DDP | 4/21 | 5/21 | 2/21 | - | - | - | - | |
| Reni 2004[19] | Gem | 9/47 | 1/47 | 2/47 | - | 4/47 | 1/47 | - |
| Gem + 5-FU + DDP + EPI | 22/52 | 15/52 | 2/52 | - | 3/52 | 2/52 | - | |
| Viret 2004[20] | Gem | 16/40 | 5/40 | 11/40 | - | 3/40 | - | - |
| Gem + DDP | 23/41 | 14/41 | 14/41 | - | 9/41 | - | - | |
| Rocha Lima 2004[21] | Gem | 54/169 | 24/169 | 22/169 | - | 31/169 | - | 3/169 |
| Gem + Irinotecan | 65/173 | 34/173 | 27/173 | - | 53/173 | - | 33/173 | |
| Costanzo 2001[22] | Gem | 1/49 | 0 | 3/49 | - | 0 | 0 | 0 |
| Gem + 5-FU | 1/41 | 1/41 | 3/41 | - | 1/41 | 2/41 | 0 | |
| Heinemann 2003[23] | Gem | 8/97 | 10/97 | 10/97 | 2/97 | 6/97 | 2/97 | 4/97 |
| Gem + DDP | 10/95 | 4/95 | 13/95 | 1/95 | 21/95 | 4/95 | 3/95 | |
| Kulke 2004[24] | Gem | 27/58 | 15/58 | 6/58 | 6/58 | 13/58 | - | 1/58 |
| Gem + DDP | 29/62 | 27/62 | 11/62 | 2/62 | 24/62 | - | 0/62 | |
| Gem + Docetaxel | 19/65 | 7/65 | 8/65 | 8/65 | 10/65 | - | 5/65 | |
| Gem + Irinotecan | 12/60 | 9/60 | 4/60 | 4/60 | 17/60 | - | 10/60 | |
| Moore2005[26] | Gem + Erlotinib | 71/282 | 28/282 | 34/282 | 45/282 | 20/282 | <1 | 17/282 |
| Gem + placebo | 73/280 | 34/280 | 34/280 | 39/280 | 20/280 | 0 | 6/280 | |
| Stathopoulos 2005[27] | Gem | 8/70 | 0/70 | 2/70 | 0 | 1/70 | 0 | 2/70 |
| Gem + Irinotecan | 10/60 | 2/60 | 2/60 | 0 | 1/60 | 0 | 2/60 | |
| Riess 2005[28] | Gem | 27/225 | 15/225 | 15/225 | 19/225 | 16/225 | - | 9/225 |
| Gem + 5-FU/CF | 26/220 | 28/220 | 18/220 | 12/220 | 30/220 | - | 8/220 | |
| Herrmann 2005[29] | Gem | 30/153 | 7/153 | 9/153 | - | 5/153 | 1/153 | 3/153 |
| Gem + Capecitabine | 34/155 | 8/155 | 9/155 | - | 8/155 | 0/155 | 8/155 |
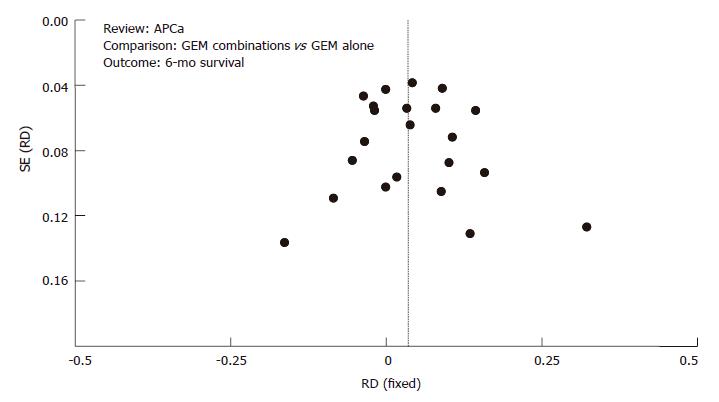
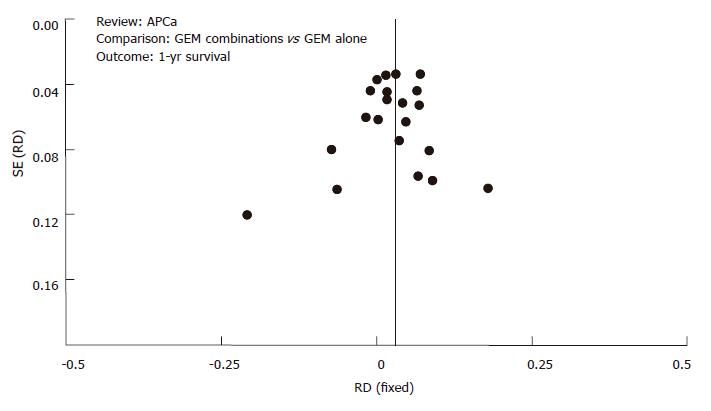
Figures 6 and 7 represent funnel plots that test for publication bias. Funnel plots for the 6-mo survival rate (Figure 6) and 1-year survival rate (Figure 7) supported the lack of evidence for publication bias.
Table 3 shows the subgroup analyses in 6-mo survival rate. It revealed that only the combined chemotherapy consisting of GEM plus a new targeted drug yielded a 6% higher survival rate as compared with chemotherapy of GEM alone.
| Subgroups | Trials | Patients | Mode | RD [95% CI] | P |
| GEM plus targeted drug vs GEM alone | [13, 14, 26] | 1496 | Fixed | 0.06 [0.01, 0.11] | 0.02 |
| GEM plus DDP vs GEM alone | [9, 10, 18, 20, 23, 24] | 560 | Fixed | 0.05 [-0.03, 0.13] | 0.24 |
| GEM plus 5-FU vs GEM alone | [12, 22, 28] | 881 | Random | 0.04 [-0.09, 0.17] | 0.57 |
| GEM plus topoisomerase I inhibitor vs GEM alone | [16, 21, 24, 27] | 928 | Fixed | 0.01 [-0.05, 0.08] | 0.72 |
| GEM plus capecitabine vs GEM alone | [8, 29] | 399 | Fixed | 0.00 [-0.08, 0.10] | 0.97 |
To improve the clinical results of GEM, Phase II-III trials have been made recently to evaluate the efficacy of combination of GEM with other drugs which were shown to be synergistic in vitro, such as 5-fluorouracil (5-FU), DDP, topotecan, etc[30,31]. Many trials demonstrated that combined GEM chemotherapy improved ORR and PFS compared with GEM alone, and a few trials reported significant OS advantage (Table 1).
The present meta-analysis shows that GEM combination produced a significant survival advantage as compared with GEM alone in patients with APCa. GEM combination was also found superior to GEM alone in terms of ORR, CBR and 6-mo TTP/PFS. Although most of the selected RCTs showed no significant survival advantage in the GEM combination group, many trials demonstrated slight survival benefit. Physicians should carefully interpret these results when they apply them in clinical practice because GEM combined with other regimens might lead to reversed therapeutic effects.
Straightforward conclusions from the results of this meta-analysis do support the use of GEM combination in patients with APCa, but toxicities from intensive chemotherapy may obliterate the survival benefit of GEM combination. In another meta-analysis, we had reported that the regimens GEM plus DDP were not superior to GEM alone in patients with APCa, which produced more side effects[32]. Furthermore, the subgroup analyses did not show any significant survival advantage in most of GEM combination groups, such as GEM plus 5-FU, GEM plus topoisomerase I inhibitor, and so on. It indicates that not all GEM combined chemotherapy have therapeutic advantage. We suggest that GEM combination, including GEM plus oxaliplatin, and GEM plus erlotinib, should be considered as optimal treatment for patients with APCa. In addition, we found that patients with good perforemance status gained great survival advantage in the sub-group analyses as reported by many other authors[28,29,12]. In our opinion, GEM combination should be applied to patients with good performance status, but carefully to the weak patients.
We found that patients receiving GEM-based combination therapy developed side effects more frequently, including neutropenia, thrombocytopenia and vomiting/nausea , which might lead to a deterioration in quality of life (QOL). However, the significant advantage of CBR and TTP/PFS in the GEM combination might be converted to the improvement of QOL. Because the primary role of chemotherapy in patients with APCa is palliative, the influence on the QOL of the patients is an important issue in determining the true value of the therapy. However, because the methods for QOL assessment from the included trials were quite different, there was no valid meta-analysis of QOL . We also noted that the CBR analysis was made in only six trials, so the result was still unreliable.
The mata-analysis was based on RCTs with high quality. We carried out a comprehensive search of the literature with barely all of cancer database. Publication bias is frequently cited as a reason for lack of validity in meta-analyses. It could occur if studies finding no association between exposure and disease were less likely to be submitted and accepted for publication than studies finding a positive association. In fact, the results of most of the studies in our meta-analyses were negative, as stated by the authors. The funnel plots also showed no evidence of publication bias. Therefore, our meta-analysis provided a valid assessment and creditable results.
Several technical issues have to be mentioned regarding this meta-analysis. One major limitation is the data source extracted from abstracted data and not individual patient data (IPD). In general, an IPD-based meta-analysis would give a more robust estimation for the association, therefore, we should interpret the results with care, especially for a positive result. Clearly, further investigations using IPD should be conducted to examine the main end points. Publication bias is a significant threat to the validity of meta-analysis. Although we detected no evidence of publication bias using the graphical method, it is difficult to completely rule out this possibility. Heterogeneity among trials can be another limitation of our meta-analysis. Although we applied a random-effect model that takes possible heterogeneity into consideration, there were still many factors causing heterogeneity, such as different drug combination, two infusion methods of gemcitabine and so on.
In conclusion, the meta-analysis indicates that GEM-based combination therapy may improve the overall survival and palliation in optimal patients with APCa as compared with GEM alone. Although the application of GEM combination is still controversial, it is a progressive method from the prospective view of point. At the same time, new regimens of drug administration should be explored in future studies.
We wish to thank Dr. Donald A Richards, F Viret, M Reni, S Raffaele, C Louvet and George P Stathopoulos for their support and data provision in our analyses.
S- Editor Wang GP L- Editor Ma JY E- Editor Ma WH
| 1. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 2. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 13036] [Article Influence: 434.5] [Reference Citation Analysis (3)] |
| 3. | Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1026] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Degen G, Hong Z. Treatment effectiveness evaluation of gemcitabine for pancreatic cancer. Zhonghua Fubu Jibing Zazhi. 2003;3:570-571. |
| 5. | Ohkawa S. Randomized controlled trial of gemcitabine in combination with UFT versus gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol. 2004;22:4131. |
| 6. | Richards DA, Oettle H, Vervenne WL, Saif MW, Thomas JP, Spitzer G, Visseren-Grul C, Enas N and Weitzman A. Randomized double-blind phase II trial comparing gemcitabine (GEM) plus LY293111 vs GEM plus placebo in advanced adenocarcinoma of the pancreas. J Clin Oncol. 2005;23:4092. |
| 7. | Shapiro J, Marshall J, Karasek P, Figer A, Oettle H, Couture F, Jeziorski K, Broome P and Hawkins R. G17DT gemcitabine [Gem] versus placebo Gem in untreated subjects with locally advanced, recurrent, or metastatic adenocarcinoma of the pancreas: Results of a randomized, double-blind, multinational, multicenter study. J Clin Oncol. 2005;23:4012. |
| 8. | Scheithauer W, Schüll B, Ulrich-Pur H, Schmid K, Raderer M, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003;14:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer. 2002;94:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Wang XY, Ni QX, Jin ML, Li ZS, Wu YX, Zhao YP and Feng FY. Gemcitabine or gemcitabine plus cisplatin for in patients with locally advanced or metastatic pancreatic cancer. Zhonghua Fubu Jibing Zazhi. 2002;24:404-407. |
| 11. | Gansauge F, Ramadani M, Pressmar J, Gansauge S, Muehling B, Stecker K, Cammerer G, Leder G, Beger HG. NSC-631570 (Ukrain) in the palliative treatment of pancreatic cancer. Results of a phase II trial. Langenbecks Arch Surg. 2002;386:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 530] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 13. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 403] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 582] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 15. | Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 718] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 16. | O'Reilly EM, Abou-Alfa GK, Letourneau R, Harker WG, Modiano M, Hurwitz H, Tchekmedyian NS, Ackerman J, De Jager RL and Eckhardt SG. A randomized phase III trial of DX-8951f (exatecan mesylate; DX) and gemcitabine (GEM) vs. gemcitabine alone in advanced pancreatic cancer (APC). J Clin Oncol. 2004;22:4006. |
| 17. | Richards DA, Oettle H, Ramanathan R, Ramanathan RK, Van Laethem J-L, Peeters M, Fuchs M, John W, Arning M and Von Hoff D. A randomized phase III study comparing gemcitabine pemetrexed versus gemcitabine in patients with locally advanced and metastatic pancreas cancer. J Clin Oncol. 2004;22:4007. |
| 18. | Li CP, Chao Y. A prospective randomized trial of gemcitabine alone or gemcitabine cisplatin in the treatment of metastatic pancreatic cancer. J Clin Oncol. 2004;22:4144. |
| 19. | Reni M, Cordio S, Passardi A, Panucci MG, Passoni P, Oliani C, Luppi G, Galli L, Nicoletti R and Villa E. Final results of a phase III trial of gemcitabine (G) versus PEFG regimen in stage IVA or metastatic pancreatic adenocarcinoma (PA). J Clin Oncol. 2004;22:4010. |
| 20. | Viret F, Ychou M, Lepille D, Mineur L, Navarro F, Topart D, Fonck M, Goineau J, Madroszyk-Flandin A and Chouaki N. Gemcitabine in combination with cisplatin (GP) versus gemcitabine (G) alone in the treatment of locally advanced or metastatic pancreatic cancer: Final results of a multicenter randomized phase II study. J Clin Oncol. 2004;22:4118. |
| 21. | Rocha Lima CMS, Rotche R, Jeffery M, Trudeau M, Cisar L A, Morganti A, Gruia G, Miller L and Green MR. A randomized phase 3 study comparing efficacy and safety of gemcitabine (GEM) and irinotecan (I), to GEM alone in patients (pts) with locally advanced or metastatic pancreatic cancer who have not received prior systemic therapy. Proc Am Soc Clin Oncol. 2003;22:1005. |
| 22. | Di Costanzo F, Sdrobolini A, Carlini P, Massidda B, Mattioli R, Iop A, Barletta E, Moscetti L, Recchia F, Tralongo P and Ospedaliera S A. Gemcitabine (GEM) Alone or in Combination with 5-FU Continuous Infusion (CI) in the Treatment of Advanced Pancreatic Cancer (APC): a GOIRC Randomized Phase II Trial. Proc Am Soc Clin Oncol. 2001;20:612. |
| 23. | Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, Neuhaus H, Haag C, Stoffregen C and Clemens M. A phase III trial comparing gemcitabine plus cisplatin vs. gemcitabine alone in advanced pancreatic carcinoma. Proc Am Soc Clin Oncol. 2003;22:1003. |
| 24. | Kulke MH, Niedzwiecki D, Tempero MA, Hollis DR and Mayer RJ. A randomized phase II study of gemcitabine/cisplatin, gemcitabine fixed dose rate infusion, gemcitabine/docetaxel, or gemcitabine/irinotecan in patients with metastatic pancreatic cancer (CALGB 89904). J Clin Oncol. 2004;22:4011. |
| 25. | Richards DA, Waterhouse DM, Wagener DJ, Krishnamurthi SS, Rosemurgy A, Dasse KD, Macdonald K, Gulyas S, Plante M, Copley-Merriman C and Grove W. Randomized, double-blind, placebo-controlled phase 2 study of the histone deacetylase inhibitor CI-994 plus gemcitabine (CI-994 G) versus placebo plus gemcitabine (P G) in the treatment of patients with advanced pancreatic cancer (APC). Proc Am Soc Clin Oncol. 2002;21:644. |
| 26. | Moore MJ, Goldstein D, Hamm J, Kotecha J, Gallinger S, Au HJ, Nomikos D, Ding K, Ptaszynski M, Parulekar W. Erlotinib improves survival when added to gemcitabine in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. 2005 Gastrointestinal Cancers Symposium: Multidisciplinary Approaches to Gastrointestinal Malignancies and Premalignancies; 2005 January 27-29; Hollywood, FL: Abstract 77. . |
| 27. | Stathopoulos G, Aravantinos G, Syrigos K, Kalbakis K, Karvounis N, Papakotoulas P, Boukovinas J, Potamianou A, Polyzos A, Christophillakis C and Georgoulias V. A randomized phase III study of irinotecan/ gemcitabine combination versus gemcitabine in patients with advanced/ metastatic pancreatic cancer. J Clin Oncol. 2005;23:4106. |
| 28. | Riess H, Helm A, Niedergethmann M, Schmidt-Wolf I, Moik M, Hammer C, Zippel K, Weigang-Köhler K, Stauch M and Oettle H. A randomised, prospective, multicenter, phase III trial of gemcitabine, 5-fluorouracil (5-FU), folinic acid vs. gemcitabine alone in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:4009. |
| 29. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Saletti P, Bajetta E, Schueller J, Bernhard J, Dietrich D and Scheithauer W. Gemcitabine (G) plus capecitabine (C) versus G alone in locally advanced or metastatic pancreatic cancer. A randomized phase III study of the Swiss Group for Clinical Cancer Research (SAKK) and the Central European Cooperative Oncology Group (CECOG). J Clin Oncol. 2005;23:4010. |
| 30. | Kanzawa F, Saijo N. In vitro interaction between gemcitabine and other anticancer drugs using a novel three-dimensional model. Semin Oncol. 1997;24:S7-8-S7-16. [PubMed] |
| 31. | Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996;2:521-530. [PubMed] |
| 32. | Xie de R, Liang HL, Wang Y, Guo SS. Meta-analysis of inoperable pancreatic cancer: gemcitabine combined with cisplatin versus gemcitabine alone. Chin J Dig Dis. 2006;7:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |