INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common tumor entity causing over one million deaths annually worldwide[1,2]. Most patients with HCC suffer two different diseases, namely a chronic and a malignant liver disease. More than 80% of all HCCs are developed in cirrhotic livers. Currently, only curative treatment options are surgical resection or liver transplantation. However, only a very few patients are eligible for these treatment options. Almost 80% of the newly diagnosed patients with HCC do not qualify for resection because of insufficient functional parenchyma or transplantation due to multi-centricity and/or other co-morbidities. Consequently, most patients unfortunately only qualify for a palliative treatment[3,4]. For these patients, a number of therapeutic interventions have been developed, termed as ablative procedures (transarterial chemoembolization, radio-frequency- or laser-induced thermo-ablation and percutaneous ethanol injection)[5]. Furthermore, several systemic chemotherapy regimes have been evaluated[6-8]. For all conservative therapies, randomised clinical trials are missing to date. Currently, chemotherapy in HCC is only applied in framework of clinical trails or arterial chemoembolization. To date, only one group has proven a survival benefit of chemoembolization in a specific subpopulation of HCC patients[9]. European Association for the Study of the Liver concluded in a consensus meeting for clinical management of HCC in 2001 that chemotherapy for HCC currently is negligible[5].
Microtubule (MT) function is essential for cell growth and interference of the dynamics of microtubule formation is considered a promising therapeutic approach in cancer treatment[10]. Unfortunately, inherent resistance to anti-microtubule agents has been found in many tumor types and acquired resistance occurs during multiple cycles of therapy. Thus, great interest lies in identifying new anti-microtubule drugs that overcome various modes of resistance. Anti-microtubule agents have not been commonly tested against HCC since initial studies had shown inherent or acquired resistance to anti-microtubule agents because of high intrinsic activity of multidrug resistance gene (MDR) in HCC[11-13].
HTI-286 is a synthetic tubulin inhibitor and a hemi-asterlin analogue. Hemiasterlin was originally identified as natural product from marine sponges. It is a potent inhibitor of cell growth, depolymerizes MTs and arrests cells in the G2-M phase of cell cycle. Loganzo et al[14] have shown that HTI-286 retains potency in cellular models resistant to several chemotherapeutics like taxanes and vinca alkaloids. HTI-286 has been fount to inhibit growth of various human tumor xenograft models, such as colon, skin, prostate, brain and breast cancer, that are resistant to currently approved anti-microtubule drugs. Additionally, HTI-286 circumvents the P-glycoprotein-mediated resistance, which hampers efficacy of several anti-microtubule agents[14,15].
Accordingly, we hypothesised that HTI-286 has a promising potential in the treatment of HCC. We investigated the proliferation inhibiting effect of HTI-286 on hepatic tumor cell lines, such as like Morris hepatoma (MH), HepG2 and Hep3B, in vitro. Primary human hepatocytes (PHH) in culture were also exposed to HTI-286 to evaluate a possible cytotoxic effect. Furthermore, in a rat allograft model, in vivo effect of intravenous HTI-286 administration on tumor growth was studied. We found that HTI-286 markedly inhibited cell proliferation of hepatocarcinoma cells in vitro and tumor growth in vivo. Interestingly, no significant cell death appeared in PHH cell culture under HTI-286 exposure.
MATERIALS AND METHODS
Compound
HTI-286 (N, β, β- trimethyl- L- phenylalanyl- N-(1S, 2E)-3-carboxy-1-isopropylbut-2-enyl N, 3 dimethyl-L-valinamide), also known as taltobulin or SPA-110, was generously provided by Dr. Frank Loganzo (Wyeth, Pearl River, NY, USA). HTI-286 was dissolved in Dulbecco’s modified Eagle’s medium (DMEM) to make two stock solutions of 5 and 0.05 mmol/L, which were then diluted in culture medium to obtain the desired concentrations.
Cell lines
Rat liver tumor Morris hepatoma 3924A (MH) cells were grown in RPMI 1640 with Glutamax I (Invitrogen) supplemented with 200 mL/L heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin, gentamycin 50 μg/mL (Invitrogen).
Human hepatocarcinoma HepG2 cells were cultured in Minimum Essential Medium Eagle with glutamine (Sigma) supplemented with 0.11 g/L sodium pyruvate and 1.5 g/L sodium bicarbonate (pH 7.2), 100 mL/L heat-inactivated FBS and 1% penicillin/streptomycin, gentamycin 50 μg/mL.
Human hepatocarcinoma Hep3B cells were cultured in DMEM (4.5 g/L glucose), Glutamax I, NaPyr (Invitrogen), 100 mL/L FBS, 1% penicillin/streptomycin and gentamycin. Isolation of primary human hepatocytes was done by a co-research group and kindly provided to us. Primary human hepatocytes were cultured in William’s medium E (Sigma) containing supplemented factors.
Evaluation of cell doubling time and determination of 50% cell growth inhibition (IC50)
Anti-proliferative activity of HTI-286 was determined in vitro using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide colorimetric method, also known as the MTT reduction assay. This assay is based on the ability of viable cells to reduce MTT-tetrazolium salt into MTT-formazan by the mitochondrial enzyme succinate-dehydrogenase[16].
Before initiating proliferation assay, cell doubling time of each tested cell line was evaluated with the MTT assay. Afterwards MH, Hep3B and HepG2 tumor cells were cultured in 96-well microtiter plates and seeded at densities predetermined. Ensuring cell confluence rates of > 50%, fresh medium with or without HTI-286 was added at different concentrations for 1, 2 or 3 doubling times. Then, 12.5 μL of an MTT solution in medium (5 mg/mL MTT; Sigma Chemical Co., St Louis, USA) was added for 3 h. The medium was removed and the MTT-formazan crystals were solubilized by adding DMSO (100 μL/well). Absorbance (A562) was determined at 562 nm. Drug concentration inhibiting 50% (IC50) growth compared to untreated cells was calculated.
Proliferation assay
MH, Hep3B and HepG2 tumor cells were cultured in 96-well microtiter plates and seeded at densities predetermined after they were starved in growth factor-depleted medium for one doubling time. Ensuring cell confluence rates of > 50%, fresh medium with or without HTI-286 was added at different concentrations for two doubling times. MTT assay was processed as described above. Results were expressed as percentage of the control. The absorbance of the control (cell culture without any treatment) corresponds to 100% MTT reduction. Three independent experiments were performed for each cell line and data were presented as mean ± SD.
DNA synthesis measurement using 5-bromodeoxyuridine (BrdU) incorporation
To evaluate the cell cycle arrest, MH cells were A405 seeded at the same densities as used for proliferation assay, but BrdU, a labelled DNA precursor (5-bromo-2’-deoxyuridine), was applied for three and half hours. After removing the medium and according to the manufacturer’s recommendations, absorbance was determined at 405 nm. The labelled precursor is incorporated into genomic DNA during the S-phase (synthesis) of the cell cycle and the detected amount is directly proportional to the rate of cell division occurring in the sample.
Immunofluorescence assay
Morris hepatoma, Hep3B and HepG2 cells were plated on cover slips placed in 6-well dishes. Two days later, different concentrations of fresh medium with or without HTI-286 were added to the wells for two cell doubling times. Cells were washed with phosphate-buffered solution (PBS), fixed for 15 min in methanol at 4°C and incubated for 1 h at room temperature with 1:500 dilution of anti-α-tubulin antibody (clone DM 1A; Sigma) in PBS. After washing with PBS, cells were incubated with 1:100 dilution of FITC-conjugated F (ab’) 2 fragment of goat anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) for 30 min at room temperature in PBS. After washing, cells were mounted in one drop of Dako Faramount (DAKO Corporation, USA). Cells were examined with an Olympus B × 61 microscope and × 40 magnification objective.
In vivo study
A total of 17 female American Cancer Institute (ACI) rats, weighing 120-145 g, were used. The official Animal Care Committee of the University of Bern approved all experimental procedures. The animals had free access to water and standard pelleted diet and were housed in a temperature-controlled room under constant 12 h light/ dark cycles. Operations were carried out under anaesthesia using clean but not sterile technique. Rats were pre-anaesthetized with ether and then put under narcosis with a mixture of dormitor, climasol and fentanyl. As antidote a mixture of antisedan, sarmasol and narcan was used. The liver was exposed via a midline incision and a single cube (1-2 mm³) of subcutaneously grown Morris hepatoma tumor was implanted between the right and left lobe. Tumors were allowed to grow for 10 d. Tumor growth was traced with magnetic resonance tomography (MRT). MRT was performed in a 1.5 Teele unit (Sonata, Siemens) using a phased array wrist coil. T2-weighted images (TSE; 3D; TRC200; TE 43 ms) in axial plane were acquired with continuous 1 mm slices (isotrope resolution).
The tumor volume was calculated according to a web-based volumetric calculator (S.D. Filip; Mississippi State University). After confirmation of successful tumor implantation, the animals were randomly divided into control and HTI-286-treated arms, each consisted of six animals, by a third person who was not involved in the study. HTI-286 was administered via tail-injection in a 0.12 mg/kg rat-weight concentration on d 1, 5, 9 and 15. The tumor growth was followed with MRI on d 7, 21, and 28 after tumor implantation. After the final MRI, animals were sacrificed under a deep narcosis.
Statistical analysis
QuickCals calculator for scientists (GraphPad Software 2002) was used for statistical analysis. Student’s t test was chosen to compare the means. Differences were considered statistically significant with two-tailed P values less than 0.05.
RESULTS
Cell doubling time and IC50
To determine a test confluence in which active cell proliferation is permitted during the experiments and also a sufficient confluence for adding the drug, the cell doubling time of each tumor cell line was first evaluated using different initial seeding densities and by following the cell growth for 7 d (data not shown). The doubling times for MH, Hep3B and HepG2 tumor cells were found to be 22 h, 43 h and 59 h, respectively. For our experiments, we chose to add the drug for two cell doubling times.
We evaluated the required IC50 concentration for each cell line with the MTT assay. All three hepatocarcinoma cell lines were exposed for 1, 2 and 3 cell doubling times to the drug. Sensitivity to HTI-286 was different in all cell lines. Mean IC50 for all tested hepatic tumor cell lines was 2 nmol/L ± 1 nmol. At 1 nmol/L, HTI-286 inhibited MH cell growth by 50%. The IC50 for Hep3B and HepG2 was 2 nmol/L and 3 nmol/L, respectively. HepG2 was the most sensitive and Hep3B the least sensitive cell line among the tested hepatocarcinoma cell lines.
Proliferation assay
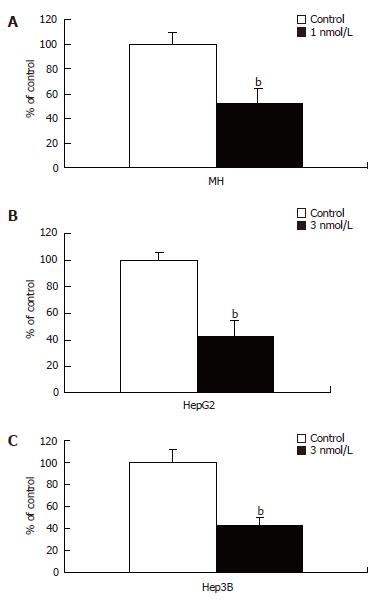
Ability of HTI-286 to inhibit cell proliferation was examined. Morris hepatoma, Hep3B and HepG2 cells were exposed at IC50 concentration to HTI-286 for two cell doubling times. All three tested hepatic tumor cell lines were significantly inhibited compared to the controls with P values of 0.005, 0.001, and 0.002 for MH, HepG2, and Hep3B, respectively (Figure 1A-C).
Figure 1 Inhibitory effect of HTI-286 on proliferation of MH, HepG2, and Hep3B cells.
Inhibitory effect upon HTI-286 treatment was highly significant in all three hepatic carcinoma cell lines. MH (A), HepG2 (B) and Hep3B (C) cells were exposed to 1, 3, and 3 nmol/L of HTI-286, respectively, for two cell doubling times. Student’s t test analysis showed two-sided P values of 0.005, 0.002 and 0.001 for MH, HepG2, and Hep3B, respectively.
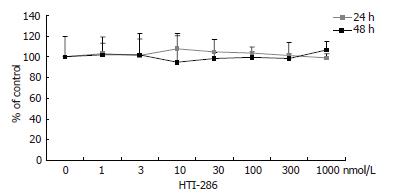
In contrast, PHH did not show an obvious decrease in viable cells in the MTT assay after continuous exposure to HTI-286 for 24 or 48 h at concentrations between 1 nmol/L and 1 mmol/L (Figure 2).
Figure 2 Effect of HTI-286 on primary human hepatocytes.
Human hepatocytes were exposed to HTI-286 for 24 and 48 h at concentrations between 1 nmol/L and 1 mmol/L. No significant decrease in viable cells was detected in MTT assay at any concentration.
These data suggest that hepatic neoplastic cells are more sensitive to HTI-286 than primary human hepatocytes. Furthermore, our data are consistent with the effects of HTI-286 on 18 different human tumor cell lines reported by Loganzo et al[14].
Effect of HTI-286 on cell cycle distribution
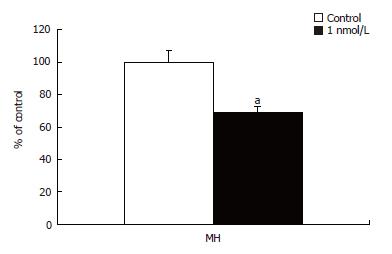
Hemiasterlin arrests cells in mitosis and induces apoptosis. HTI-286-treated MH cells were analysed by 5-bromodeoxyuridine (BrdU) incorporation assay. Cells showed significant decrease in the S phase when being exposed to HTI-286 at 1 nmol/L for two cell doubling times. DNA synthesis was significantly reduced in the treated cells (P = 0.02) indicated by reduced BrdU incorporation compared to the untreated cells (Figure 3). With increasing concentration, more cells were found to be arrested into this phase (data not shown). Loganzo et al[14], using fluorescence-activated cell sorting analysis (FACS), identified the cell cycle arrest in the G2-M phase. However, we did not have the possibility to perform a FACS analysis. These data are consistent with reported effect of hemiasterlin and HTI-286 on cell cycle and cell proliferation.
Figure 3 BrdU incorporation in MH cells treated with 1 nmol/L HTI-286.
Decrease in S phase was observed as BrdU incorporation decreased significantly (P = 0.024) in MH cells compared to the untreated cells when exposed to 1 nmol/L HTI-286 for two cell doubling times.
Effect of HTI-286 on microtubule structure and cell morphology
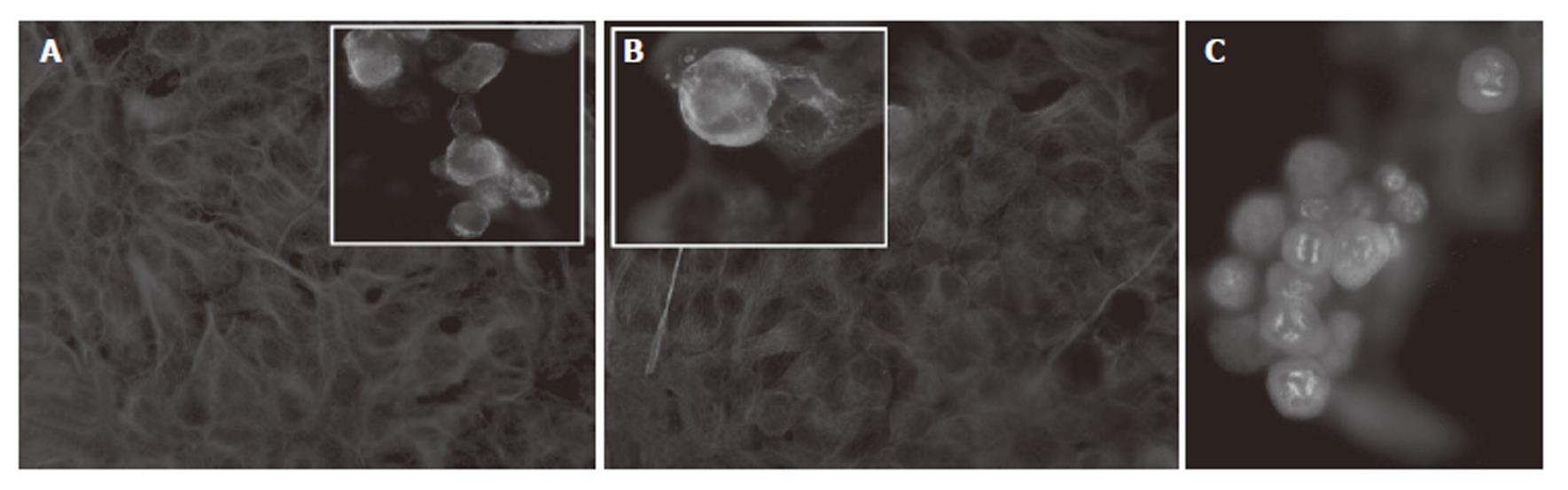
The effect of HTI-286 on microtubule structure was examined by immunofluorescence microscopy using an antibody specific for α-tubulin. Untreated MH, Hep3B and HepG2 cells contained a dense and complex network of MTs in general extending from the perinuclear region to the cell periphery. MH, Hep3B, and HepG2 cells were exposed to different concentrations (1 nmol/L-30 nmol/L) of HTI-286. Microtubule structure was disrupted by HTI-286 at low concentrations (Figure 4A and B). Furthermore, cell morphology changed during drug exposure. Cell rounding and swelling phenomena were the most significant attributes accompanied by a decrease in cell number. Accumulation of cells in metaphase was seen (data not shown). With increasing concentration of HTI-286, diffuse tubulin staining was evident with loss of cell adhesion, indicating a general disruption of cellular integrity. Additionally, multipolar spindles appeared, indicating disturbed spindle function in cells undergoing mitosis (Figure 4C). These data confirm that HTI-286 permeates hepatocarcinoma cells and modify MT structure and are consistent with the previous reported effect of HTI-286 on tubulin.
Figure 4 Microtubule (MT) structure assessed by immunofluorescene microscopy with a specific α-tubulin antibody.
A and B: Untreated Hep3B and HepG2 cells showed dense and complex MT network in the cytoplasm. Insets A and B represent cells after HTI-286 treatment at 3 nmol/L for two cell doubling times. Decreased cytoplasmic microtubule density and diffuse staining were observed. Magnification x 40 except for insets, which are magnified further to outline the morphological changes. C: MH cells treated with HTI-286 at 1 nmol/L for two cell doubling times showed multipolar spindles, indicating disruption of the microtubule network in mitotic cells.
In vivo efficacy of HTI-286
Morris hepatoma tumors were implanted into the liver of rats as described in methods. The perioperative mortality (tumor implantation) was zero percent. Successful tumor growth was seen in almost 70% of the animals on 10th post-implantation day. Of those animals with good tumor development, 12 were randomized into two groups, each consisting of six animals. One group was given 0.12 mg/kg of HTI-286 intravenously on d 1, 5, 9 and 15. The dose was chosen according to advice by Wyeth. Rats tolerated intravenous HTI-286 treatment well. No clinical signs of hepatic or renal insufficiency were seen in the animals. The control group was not given any specific treatment but received the vehicle and the natural tumor progression was followed. The tumor volume at randomization did not differ between both groups (P = 0.14). Two animals from the control group died during the second MRI examination due to anaesthesia complications and were excluded from the analysis. By d 7, after only two doses of HTI-286, tumor growth was significantly inhibited in the treated group compared to the control group (P = 0.04). The last dose of HTI-286 was applied on d 15. The growth difference on d 21 reached high significance after completion of treatment (4 doses in total) in the HTI-286-treated group compared to the control group (P = 0.0001). Tumor growth was followed for one week more without application of HTI-286. On d 28, the MRI still revealed smaller tumor in the treated group, indicating lasting inhibitory effect; however, the difference did not reach statistical significance (P = 0.056) (Figure 5).
Figure 5 In vivo inhibition of tumor growth under HTI-286 treatment.
HTI-286 was injected on d 1, 5, 9 and 15. By d 7, tumor growth significantly differed between the control and HTI-286 group (aP = 0.042; bP = 0.0001). Two weeks after the last HTI-286 injection, the inhibitory effect was diminished between both groups (P = 0.056).
DISCUSSION
HCC is the most common cause of death due to cancer in some areas of Asia and with steadily rising incidence in Europe and USA as well. In Europe, it is already the primary cause of death in patients with cirrhosis[17]. Late presentation and lack of systemic therapy result in median survival of less than 12 mo. Less than 25% of HCC patients qualify for resection or transplantation[18]. To date, no chemotherapy regimen has been established for patients with unresectable HCC. Ultimately, the majority of HCC patients can only be treated symptomatically and they have dismal prognosis[19].
The few studies of anti-microtubule agents used for the treatment of HCC have shown high intrinsic multidrug resistance (MDR) gene activity, resulting in ATP-dependent export of chemotherapeutic drugs across the plasma membrane mediated by transporters of the MDR P-glycoprotein family (P-gp). Tubulin binding agents are typically good substrates for P-gp and may be partially responsible for the poor response rates observed in HCC patients[20-22].
Hemiasterlins are a family of potent cytotoxic peptides initially isolated from marine sponges. Anderson et al[15] have reported in vitro and in vivo anticancer activity of hemiasterlins and clarified underlying mechanisms of cytotoxicity. HTI-286 is a synthetic analogue of naturally occurring hemiasterlins and a potent antimitotic agent. In 18 different human tumor cell lines, HTI-286 has been proven as a potent inhibitor of cell proliferation and having substantially less interaction with P-gp than currently used taxanes and vinca alkaloids. HTI-286 has been shown significantly effective in tumors resistant to paclitaxel and vincristine. Furthermore, in human tumor xenografts, HTI-286 inhibited growth of tumors derived from colon, brain, skin, prostate, and breast[14].
It has been shown that neoplastic cells are either inherently refractory to tubulin binding agents (taxanes and vinca alkaloids) or acquire resistance during repetitive therapy[23-25]. Such resistance, whether inherent or acquired, is associated with induction of signal transduction pathways, expression of MDR, differential tubulin isotypes, tubulin mutations, interaction of tubulin with the cytoskeleton, and alteration of apoptosis-regulating proteins, such as p53, bcl family members, or survivin[10,26,27].
Our data demonstrate that HTI-286 is an effective inhibitor of hepatic tumor cell proliferation in vitro. The mean IC50 of HTI-286 in our experiments (2 nmol/L ± 1 nmol/L) is consistent with the reported IC50 of 2.5 nmol/L reported by Loganzo et al[14]. Anderson et al[15] reported IC50 for hemiasterlin A and B of 0.5 and 2 nmol/L, respectively. With intravenous HTI-286 treatment, growth of Morris hepatoma tumors was also significantly inhibited. However, two weeks after the last intravenous dose, the inhibitory effect diminished. These findings are also consistent with the in vivo efficacy reported by Loganzo et al[14].
The data shown here demonstrate sensitivity of hepatic tumor cells to HTI-286. The observed differences in sensitivity and viability between the tested tumor cell lines and PHH may be related to different tubulin isotype expression in malignant versus normal parenchymal cells. Although human hepatocytes do not proliferate in culture and lose their hepatic-specific metabolic activity and differentiation irreversibly after being maintained in culture for 5 to 7 d, our data suggest a less cytotoxic effect of HTI-286 on PHH compared with hepatic tumor cells[28,29]. Interestingly, no significant decrease in viable cells was observed at concentrations from 1 nmol/L to 1 mmol/L in PHH culture. In contrast, 50% growth inhibition was observed in MH, Hep3B, and HepG2 cells exposed to only 1-3 nmol/L HTI-286. However, the cell metabolism of human hepatocytes under HTI-286 has not been evaluated.
Six different alpha- and beta-tubulins have been described to date[30,31]. Furthermore, the six isotypes are sub-classified according to their C-terminal composition, which is highly divergent. In addition, post-translational modifications enhance the divergence[32,33]. Several studies have shown a positive correlation between expression of beta-tubulin isotype and resistance to paclitaxel and docetaxel. Accordingly, alterations of alpha-tubulin have been described resulting in resistance to tubulin binding agents as well[34-38]. Currently, only one study is available on expression of tubulin isotypes in HCC, response rate and resistance to vinca alkaloids and taxanes[39].
Microtubules are in dynamic equilibrium with the pool of soluble tubulin dimers in the cell, with constant incorporation and release of the dimers into the polymerized structures and back into the soluble tubulin pool[40]. Microtubule dynamic (MD) is the phenomenon of switching between growing and shortening states of MT ends. In proliferating and neoplastic cells, MD is increased[41,42]. Microtubule dynamic is suppressed by HTI-286[43]. Drugs targeting tubulin induce apoptosis. In the case of tubulin- binding agents, although the mechanisms have not yet been completely clarified, it seems that inhibition of MD is closely associated with changes in the expression profile and functional status of apoptotic/anti-apoptotic-regulatory molecules and MT-associated proteins[34,44,45]. Additionally, drug-resistance to tubulin-binding agents is also linked to cytoprotective and anti-apoptotic factors, such as p53, bcl-2, or bcl-xL. Current molecular understanding of the mechanisms of cell death by tubulin-targeting agents suggests that these agents inhibit MD and cell cycle G2/M-phase transition, which triggers molecular signalling that induces mitochondrial permeability and the release of pro-death molecules into the cytosol inducing caspase-dependent apoptosis[46,47]. Giannakakou et al[48] showed suppression of MD with low concentrations of MT-targeting compounds enhancing microtubule-dependent trafficking and accumulation of p53 into the nucleus and activation of p53-downstream target genes as well as induction of p53-upregulated modulators of apoptosis. Evidence is strong that MDR1 expression is negatively correlated with p53, and MDR1 has been shown to be responsible for drug resistance to tubulin-binding agents[49]. HTI-286 is a very poor substrate for MDR1 encoded drug-efflux pump. p53 directly affects the MD by transcriptional regulation of proteins involved in modulation of microtubules, known as MT-associated proteins (MAPs). Bcl family members have been identified as transcriptional targets of p53. Anti-apoptotic function of bcl2 and pro-apoptotic function of bax depend on their dimerization status. Furthermore, anti-microtubule agents induce bcl2 inactivation through phosphorylation, which parallels their inability to form heterodimers with bax. Consequently, bax-bax homodimer levels rise and evoke apoptosis[50]. These findings are supported by the fact that bcl2 phosphorylation appears at G2-M phase[51]. Microtubule-targeting drugs cause cell cycle arrest in G2-M phase. Although the alteration of the molecular environment during HTI-286 exposure has not yet been evaluated, it is likely that the aforementioned molecular mechanisms leading to apoptosis also apply to HTI-286-induced cell death. HTI-286 inhibits proliferation at very low concentrations, suggesting that molecular events are impacted at low drug concentrations. Notably, suppression of MD is the most decisive factor in initiation of apoptosis and that all types of MT-targeting drugs, independent of their class (depolymerizing or polymerizing agents), modulate MD.
In addition to mitosis, tubulin plays an important role in the interphase cell in maintaining the celluar subcompartiments and distribution of organelles (mitochondria, Golgi, endoplasmis reticulum, lysosomes, endosomes and nucleus)[52-57]. This finding suggests a complex role for tubulin in signal transduction and transcription. Our α-tubulin staining data implicate a complex network of MT in the interphase cell and a role in cellular organisation and metabolism.
In conclusion, our data suggest that HTI-286 is highly effective in inhibiting hepatic tumor cell proliferation in vitro and tumor growth in vivo. Primary human hepatocytes appear to be unaffected by high doses of HTI-286. Further investigation of HTI-286 in patients with advanced HCC or with primary unresectable HCC is warranted. Currently, HTI-286 is in clinical development.