Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6674
Revised: September 12, 2006
Accepted: September 27, 2006
Published online: November 7, 2006
AIM: To investigate the incidence and risk factors of late-onset acute rejection (LAR) and to clarify the effectiveness of our immunosuppressive regime consisting of life-long administration of tacrolimus and steroids.
METHODS: Adult living donor liver transplantation recipients (n = 204) who survived more than 6 mo after living donor liver transplantation were enrolled. Immunosuppression was achieved using tacrolimus and methylprednisolone. When adverse effects of tacrolimus were detected, the patient was switched to cyclosporine. Six months after transplantation, tacrolimus or cyclosporine was carefully maintained at a therapeutic level. The methylprednisolone dosage was maintained at 0.05 mg/kg per day by oral administration. Acute rejections that occurred more than 6 mo after the operation were defined as late-onset. The median follow-up period was 34 mo.
RESULTS: LAR was observed in 15 cases (7%) and no chronic rejection was observed. The incidence of hyperlipidemia, chronic renal failure, new-onset post-transplantation diabetes, and deep fungal infection were 13%, 2%, 24%, and 17%, respectively. Conversion from tacrolimus to cyclosporine was required in 38 patients (19%). Multivariate analysis revealed that a cyclosporine-based regimen was significantly associated with LAR.
CONCLUSION: Both LAR and drug-induced adverse events happen at a low incidence, supporting the safety and efficacy of the present immunosuppression regimen for living donor liver transplantation.
- Citation: Akamatsu N, Sugawara Y, Tamura S, Keneko J, Matsui Y, Hasegawa K, Makuuchi M. Late-onset acute rejection after living donor liver transplantation. World J Gastroenterol 2006; 12(41): 6674-6677
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6674
Standard regimens for immunosuppressive therapy after liver transplantation include calcineurin inhibitors and steroids, which result in a reduced incidence of acute rejection and improved recipient survival[1]. The long-term complications of chronic immunosuppression, such as diabetes mellitus, renal toxicity, hyperlipidemia, and opportunistic infections, however, are a great concern with regard to improved survival.
Because the majority of acute rejections occur within the first few months after liver transplantation, most centers try to taper off steroids and minimize maintenance trough levels of calcineurin inhibitors within the first 6 mo after liver transplantation[2,3]. Recently, more rapid steroid withdrawal programs (within 2 wk) were introduced[4,5]. The shorter steroid regimen increases the possibility of late-onset acute rejection (LAR), which might result in graft loss and serious morbidity[6].
The appropriate doses of immunosuppressive drugs over the long-term should be balanced against the incidence of LAR and drug complications. Our immunosuppressive regimen after adult living donor liver transplantation (LDLT) consists of life-long administration of steroids and tacrolimus strictly controlled with therapeutic drug monitoring. In the present study, we retrospectively investigated the incidence and risk factors of LAR to clarify the safety and efficacy of our immunosuppressive regime.
A total of 247 LDLTs were performed in adult patients at the University of Tokyo Hospital between January 1996 and March 2005. Among the 224 recipients, 18 patients were excluded because of death within the 6 mo. Moreover, another two cases were excluded, one who received the graft from his identical twin and another who received auxiliary partial orthotopic liver transplantation. The remaining 204 patients (102 men and 102 women; age range: 18-67 years) were enrolled in the study. The median postoperative follow-up period was 34 mo (range, 6-114). The most common indication for LDLT was viral cirrhosis (n = 77) followed by primary biliary cirrhosis (n = 46).
Pre-operative aspartate transaminase, total bilirubin levels, and serum creatinine were 19-308 IU/L, 4-400 mg/L, and 2-44 mg/L, respectively. The median score for model for end-stage liver disease was 14 (range, 4-34).
Our surgical technique for recipient and donor surgery is described elsewhere[7]. All the patients received the same immunosuppressive regimen (Table 1) consisting of tacrolimus (FK, Prograf, Astellas Pharma Inc., Tokyo, Japan) and methylprednisolone. If adverse effects occurred with FK, the patient was switched to cyclosporine (CsA). The indications for conversion are described elsewhere[8]. More than 6 mo after LDLT, FK and CsA were maintained at 5 to 10 μg/L and 100 to 150 μg/L, respectively, with therapeutic drug monitoring at least once a month. A trough level of less than 5 μg/L FK or less than 100 μg/L CsA was regarded as sub-therapeutic. Methylprednisolone was maintained at 0.05 mg/kg per day by oral administration for lifetime of the recipients after the initial 6 mo.
| Postoperativetime (d) | Tacrolimus(μg/L) | Cyclosporine(μg/L) | Methylprednisolone(mg/kg daily) |
| 1-7 | 15-20 | 300-350 | 20-0.75 |
| 8-14 | 14-16 | 250-300 | 0.5-0.3 |
| 15-90 | 10-15 | 200-250 | 0.3-0.12 |
| 91-180 | 8-10 | 150-200 | 0.08-0.12 |
| 180- | 5-10 | 100-150 | 0.05 |
Diagnosis of acute rejection was based on internationally accepted histologic criteria[9]. Acute rejection occurring more than 6 mo after LDLT was regarded as LAR. Biopsy-proven acute cellular rejection scored more than 3 in Banff classification was treated with high-dose methylprednisolone (20 mg/kg per day) followed by recycling. Patients with steroid-resistant cellular rejection were treated with mycophenolate mofetil and anti-T-cell monoclonal antibody (OKT3, Ortho-Biotech Corporation, Raritan, NJ).
The cytomegalovirus status of the patient was moni-tored by pp65 antigenemia assay once a week for 3 mo postoperatively. When there were more than 5 antigen-positive cells/50 000 white blood cells, antiviral therapy was started until the antigenemia assay was negative. Details of antibacterial and antifungal prophylaxis are described elsewhere[10].
Hyperlipidemia was diagnosed when the serum cholesterol level was over 2300 mg/L in two successive examinations. Chronic renal failure was defined as serum creatinine of more than 25 mg/L on at least two successive determinations more than 6 mo after LDLT. Patients with normal glucose tolerance preoperatively, who required pharmacologic assistance to control blood sugar more than 6 mo after the transplantation, were diagnosed with new-onset post-transplantation diabetes.
The incidence of acute rejection and post-transplantation complications more than 6 mo after LDLT was examined. Preoperative factors included age, gender, disease, serum total bilirubin levels, model for end-stage liver disease score, donor/recipient blood type, gender match, donor age, and the result of donor/recipient human leukocyte antigens (HLA) and the lymphocytotoxic crossmatch. Intraoperative factors included anhepatic phase duration, blood loss, and graft weight/standard liver volumes[11]. Postoperative factors were initial immunosuppressive regimen (FK or CsA), cytomegalovirus infection, and episodes of early acute rejection.
Categorical data were compared using chi-square or Fisher’s exact tests. Continuous data were compared using the t-test or Mann Whitney U test. Multiple regression analysis was performed using the proportional hazards models to identify factors that were independently associated with LAR. The trough levels of the calcineurin inhibitors 6 mo after LDLT were recorded and compared between patients with LAR and those without. LAR-free survival was stratified by immunosuppressive regimen using the log-rank test. A P value of less than 0.05 was considered statistically significant. Values of measured variables were expressed as median and range.
None of the patients experienced immediate graft non-function or chronic rejection. Postoperative vascular, hemorrhagic, and biliary complications that required re-operation within 6 mo, occurred in 75 patients (37%). Conversion from FK to CsA was required in 38 patients (19%) during the initial 6 mo after surgery. The most common reason for conversion was neurotoxicity (n = 15), followed by hematopoietic disorder (n = 7), diabetes mellitus (n = 6), gastrointestinal intolerance (n = 4), hepatotoxicity (n = 3) and cardio-pulmonary disorder (n = 3). The median time to conversion was 16 d (range: 6-165). The cumulative 1- and 3-year patient survival rates were 90% and 87%, respectively.
Serum cholesterol and serum creatinine levels were 1130 mg/L (range, 560-2110) and 10 mg/L (range, 2-44) preoperatively, and 1850 mg/L (range, 900-3760) and 8.6 mg/L (range, 3-31) at 6 mo after transplantation, respectively. The incidence of hyperlipidemia, chronic renal failure, and new-onset post-transplantation diabetes was 13% (n = 27), 2% (n = 3), and 24% (n = 48), respectively. To date, none of the patients has developed symptomatic cardiovascular diseases or end-stage renal disease that required hemodialysis or kidney transplantation.
Positive cytomegalovirus antigenemia occurred in 40% (82/204), all within 6 mo of surgery. The incidence of deep fungal infection that occurred more than 6 mo after surgery was 17% (35/204), which included candidiasis (n = 20), aspergillosis (n = 5), Pneumocystis carinii pneumonia (n = 3), and cryptococcosis (n = 2). The median time to the diagnosis was 192 d (range: 181-326). These deep infections were all successfully treated, except for one patient who died due to cryptococcosis.
LAR was observed in 15 cases (7%, Table 2). The median time to LAR was 302 d (range: 182-1490). All LAR cases were on a maintenance dose of steroid (methylprednisolone 0.05 mg/kg per day), except for one patient who had undergone steroid withdrawal 10 mo before LAR because of aseptic necrosis of the femoral head. Univariate analysis revealed that CsA-based immunosuppressive regimen at the onset of LAR and lower recipient age were significantly associated with LAR (Table 2). Multivariate analysis revealed that only the CsA-based regimen was an independent predictor (Hazard ratio, 0.033; range, 0.007 to 0.142; P < 0.0001).
| Factors | Variables (n) | % LAR | P |
| Preoperative | |||
| Age (yr) | < 40 (53) vs ≥ 40 (151) | 12 vs 6 | 0.03 |
| Gender | Men (102) vs women (102) | 8 vs 8 | 0.79 |
| Disease: Viral | Yes (77) vs No (127) | 9 vs 6 | 0.41 |
| Total bilirubin (mg/L) | < 50 (100) vs ≥ 50 (104) | 8 vs 7 | 0.72 |
| MELD score | < 10 (123) vs ≥ 10 (81) | 7 vs 9 | 0.57 |
| Blood type match | Identical (163) vs compatible (41) | 8 vs 5 | 0.5 |
| Gender match | Yes (91) vs No (113) | 9 vs 6 | 0.48 |
| Donor age (yr) | < 40 (84) vs ≥ 40 (120) | 10 vs 6 | 0.32 |
| HLA-A mismatch (n) | 0 (75) vs 1 or 2 (128) | 9 vs 6 | 0.64 |
| HLA-B mismatch (n) | 0 (38) vs 1 or 2 (166) | 11 vs 7 | 0.41 |
| HLA-DR mismatch (n) | 0 (43) vs 1 or 2 (161) | 5 vs 8 | 0.44 |
| T-LCX | Negative (195) vs positive (9) | 8 vs 0 | 0.39 |
| B-LCX | Negative (116) vs positive (88) | 5 vs 7 | 0.71 |
| Operative | |||
| Anhepatic time (min) | < 150 (116) vs ≥ 150 (88) | 8 vs 7 | 0.8 |
| Blood loss (mL/kg) | < 100 (88) vs ≥ 100 (116) | 7 vs 8 | 0.8 |
| Graft weight/SLV (%) | < 50 (69) vs ≥ 50 (135) | 7 vs 7 | 0.97 |
| Postoperative | |||
| Immunosuppressive regimen | FK (166) vs CsA (38) | 32 vs 2 | < 0.0001 |
| Proceeding CMV infection | Yes (82) vs No (116) | 7 vs 8 | 0.91 |
| Early acute rejection | Yes (61) vs No (143) | 11 vs 6 | 0.14 |
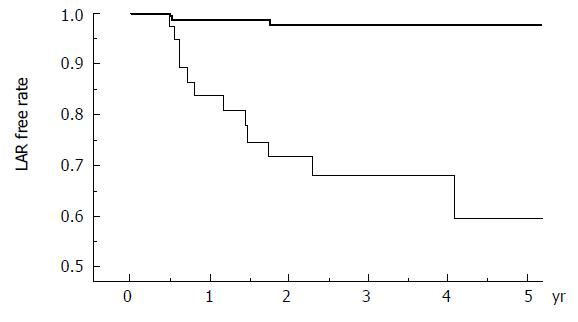
LAR-free survival stratified by immunosuppression regimen (FK-based or CsA-based) is shown in Figure 1. In those who developed LAR, only one patient in the CsA group had a sub-therapeutic level at least once during the preceding 8 wk. The trough levels more than 6 mo after transplantation were properly maintained within the target range in both FK-based and CsA-based recipients and were not related to LAR (Table 3). All LAR patients were successfully treated with steroid recycle therapy only, except for two who required additional mycophenolate mofetil and anti-T-cell monoclonal antibody administration. None of them developed chronic rejection.
| Group | All | LAR | No LAR | P |
| Mean (range) | Mean (range) | Mean (range) | ||
| Tacrolimus (μg/L) | 7.0 (4.5-11.3) | 7.4 (6.5-9.2) | 7.0 (4.5-11.3) | 0.47 |
| Cyclosporine (μg/L) | 133 (70-210) | 135 (79-210) | 128 (70-190) | 0.35 |
In the present study, there was a 7% incidence of LAR, which is an acceptable rate compared with previous studies (7%-23%)[12-16]. The incidence of early acute rejection in this population was 30% (61/204), and HLA-DR mismatching and positive T-lymphocytotoxic crossmatch had been proved to be independent significant predictors of early acute rejection[17]. Neither HLA compatibility nor lymphocytotoxic crossmatch was associated with LAR. Episodes of early acute rejection were not related to LAR, as reported previously[12,15]. The present study indicated that a CsA-based immunosuppressive regimen and lower recipient age were significant risk factors for LAR, which is consistent with previous studies[18].
Whether FK or CsA is the primary immunosuppressant depends on the transplant center, although FK is currently prescribed for nearly 90% of new liver transplantation recipients[1]. In recent randomized trials in adults, there was no difference in the incidence of acute rejection over 1 to 30 mo[19]. It indicates that CsA and FK are equivalent immunosuppressants when maintained at high therapeutic levels. Previous reports emphasized that sub-therapeutic levels of calcineurin inhibitors are related to LAR[12-15], which was not supported by the present data (Table 3). Based on the properly maintained trough level of FK and CsA in our series, the patient drug-compliance bias is negligible and the higher LAR incidence in the CsA group might represent incomplete immunosuppression in the present CsA-based regimen especially for young recipients.
Steroid withdrawal after liver transplantation is still controversial. There are reports of successful steroid withdrawal in randomized studies[2,5]. In contrast, Pageaux et al[4] reported a higher incidence of rejection in a steroid withdrawal group. Yoshida et al[14] reported that low-dose steroids increased the incidence of LAR. The aim of steroid withdrawal is to reduce long-term complications, such as diabetes mellitus, hyperlipidemia, and opportunistic infections. When compared to steroid free protocols our results with steroid maintenance showed comparable incidence in opportunistic infection, normal serum creatinine and cholesterol level (6-12 mg/L and 1290-2320 mg/L, respectively) although incidence of postoperative diabetes mellitus (24%) seemed higher. Our protocol of life-long steroid maintenance might be justified by the lower incidence of LAR without increased risk of opportunistic infections and metabolic complications.
In conclusion, an FK-based regimen with life-long steroid maintenance (0.05 mg/kg per day) is safe and effective after liver transplantation. In young (< 40 years of age) recipients who require conversion from FK to CsA, careful observation for LAR is necessary.
| 1. | Busuttil RW, Lake JR. Role of tacrolimus in the evolution of liver transplantation. Transplantation. 2004;77:S44-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Greig P, Lilly L, Scudamore C, Erb S, Yoshida E, Kneteman N, Bain V, Ghent C, Marotta P, Grant D. Early steroid withdrawal after liver transplantation: the Canadian tacrolimus versus microemulsion cyclosporin A trial: 1-year follow-up. Liver Transpl. 2003;9:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Pirenne J, Aerts R, Koshiba T, Van Gelder F, Roskams T, Schetz M, Verhaegen M, Lauwers P, Fevery J, Nevens F. Steroid-free immunosuppression during and after liver transplantation--a 3-yr follow-up report. Clin Transplant. 2003;17:177-182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, Samuel D. Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl. 2004;10:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, Trunecka P, Krawczyk M, Clavien PA, Ducerf C. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transpl. 2005;11:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immuno-suppression in liver transplant recipients. Transplantation. 1997;63:243-249. [RCA] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Dulundu E, Sugawara Y, Makuuchi M. Revolution and refinement of surgical techniques for living donor partial liver transplantation. Yonsei Med J. 2004;45:1076-1088. [PubMed] |
| 8. | Tamura S, Sugawara Y, Kishi Y, Akamatsu N, Kaneko J, Murai N, Makuuchi M. Conversion to cyclosporine provides valuable rescue therapy for living donor adult liver transplant patients intolerant to tacrolimus: A single-center experience at the University of Tokyo. Transplant Proc. 2004;36:3242-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Terminology for hepatic allograft rejection. International Working Party. Hepatology. 1995;22:648-654. [PubMed] |
| 10. | Akamatsu N, Sugawara Y, Kaneko J, Kishi Y, Makuuchi M. Risk factors of cytomegalovirus infection after living donor liver transplantation. Hepatogastroenterology. 2005;52:197-199. [PubMed] |
| 11. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 710] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation. 1992;54:821-824. [RCA] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995;60:1098-1103. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yoshida EM, Shackleton CR, Erb SR, Scudamore CH, Mori LM, Ford JA, Eggen H, Wynn V, Partovi N, Keown PA. Late acute rejection occurring in liver allograft recipients. Can J Gastroenterol. 1996;10:376-380. [PubMed] |
| 15. | Ramji A, Yoshida EM, Bain VG, Kneteman NM, Scudamore CH, Ma MM, Steinbrecher UP, Gutfreund KS, Erb SR, Partovi N. Late acute rejection after liver transplantation: the Western Canada experience. Liver Transpl. 2002;8:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Florman S, Schiano T, Kim L, Maman D, Levay A, Gondolesi G, Fishbein T, Emre S, Schwartz M, Miller C. The incidence and significance of late acute cellular rejection (> 1000 days) after liver transplantation. Clin Transplant. 2004;18:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Sugawara Y, Makuuchi M, Kaneko J, Saiura A, Imamura H, Kokudo N. Risk factors for acute rejection in living donor liver transplantation. Clin Transplant. 2003;17:347-352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | O'Grady JG, Burroughs A, Hardy P, Elbourne D, Truesdale A. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002;360:1119-1125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Fisher RA, Stone JJ, Wolfe LG, Rodgers CM, Anderson ML, Sterling RK, Shiffman ML, Luketic VA, Contos MJ, Mills AS. Four-year follow-up of a prospective randomized trial of mycophenolate mofetil with cyclosporine microemulsion or tacrolimus following liver transplantation. Clin Transplant. 2004;18:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Zhu LH E- Editor Ma WH