Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6585
Revised: August 12, 2006
Accepted: September 2, 2006
Published online: November 7, 2006
Celiac disease (CD) is a common autoimmune disorder, induced by the intake of gluten proteins present in wheat, barley and rye. Contrary to common belief, this disorder is a protean systemic disease, rather than merely a pure digestive alteration. CD is closely associated with genes that code HLA-II antigens, mainly of DQ2 and DQ8 classes. Previously, it was considered to be a rare childhood disorder, but is actually considered a frequent condition, present at any age, which may have multiple complications. Tissue transglutaminase-2 (tTG), appears to be an important component of this disease, both, in its pathogenesis and diagnosis. Active CD is characterized by intestinal and/or extra-intestinal symptoms, villous atrophy and crypt hyperplasia, and strongly positive tTG auto-antibodies. The duodenal biopsy is considered to be the “gold standard” for diagnosis, but its practice has significant limitations in its interpretation, especially in adults. Occasionally, it results in a false-negative because of patchy mucosal changes and the presence of mucosal villous atrophy is often more severe in the proximal jejunum, usually not reached by endoscopic biopsies. CD is associated with increased rates of several diseases, such as iron deficiency anemia, osteoporosis, dermatitis herpetiformis, several neurologic and endocrine diseases, persistent chronic hypertransami-nasemia of unknown origin, various types of cancer and other autoimmune disorders. Treatment of CD dictates a strict, life-long gluten-free diet, which results in remission for most individuals, although its effect on some associated extraintestinal manifestations remains to be established.
- Citation: Rodrigo L. Celiac disease. World J Gastroenterol 2006; 12(41): 6585-6593
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6585.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6585
Celiac disease (CD) is an immune-mediated disorder, the only one with a well-established origin, resulting from a permanent gluten intolerance, which primarily involves the gastrointestinal tract.
It is characterized by the presence of chronic infla-mmation of the small bowel’s mucosa and submucosa, and is clinically characterized by the presence of diverse systemic manifestations.
It may start at any age, both during childhood and adolescence, and is also relatively common in adulthood. It is being increasingly diagnosed even in elderly patients (up to 20% of patients are older than 60 years, at the time of diagnosis)[1-3].
The causal agent, gluten, is well established; a mixture of proteins present exclusively in cereals –mainly wheat, barley, rye and oats- but not corn. It affects only predisposed individuals, whose most susceptible genetic features are related to human leukocytes antigens from class two (HLA-II), predominantly HLA-DQ2 (90%), while much less frequency HLA-DQ8 (5%-10%). However, these genetic markers are a necessary, but not a sufficient condition, since a significant frequency of CD patients (5%-10%) are DQ2 and DQ8-negative. This means that other, still not well-established, genotypes may exist that probably correspond to the class one HLA system (HLA-I), including MICA, MICB and others[4].
A strong genetic susceptibility is present with about 75% concordance rate, among monozygotic twins. Certain populations have an increased prevalence of CD. For instance, the first-degree relatives of individuals with biopsy-proven CD, have a prevalence between 4%-12% of those suffering from this disease. Second-degree relatives also appear to have an increased prevalence. Patients with type 1 diabetes mellitus (IDDM) have a prevalence of CD ranging to 3%-8%. In Down´s syndrome, the presence of CD is found between 5%-12%. Also, CD is associated with Turner’s and William’s syndromes, IgA deficiency and several autoimmune disorders[5-7]. Most affected individuals show sustained clinical remission when they are put strictly on a gluten-free diet (GFD), which must be maintained indefinitely due to this illness’ genetic background.
Until the last decade, CD was considered to be a rare disease, but today it is known to be universally distributed, to involve all races, and it is one of the most commonly known genetic diseases, with a mean prevalence of 1%-2% in the general population, being clearly underestimated and underdiagnosed worldwide.
This would represent an estimated 3 million people affected in Europe and at least another 3 million in the United States. Selected ethnic groups may have a lower prevalence than that suggested for the Caucasian race, but not far from them, as the worldwide distribution of CD is pretty homogeneous, the only exception being the Saharawui population, which lives in refugee camps. This population in North Africa has a mean estimate of 5%, the highest found all over the world[8-10].
In Southern Asia there are still no data available of CD prevalence in the general population. This fact may be due to partly, because in past decades the reports of gluten intolerance were only of sporadic cases. Nowadays, several recent studies suggest that CD is also common in South Asia. For instance, 26%-49% of Indian children presenting with chronic diarrhea, are diagnosed with CD in tertiary care hospitals[11,12].
If we assume that China has approximately 1 billion inhabitants, we can extrapolate the same world proportion of CD (around 1% of the general population), then the approximate number of possible celiac patients in this country would be around ten million people. There are few reports of CD in Far East countries, yet recently, three adult cases have been reported in Canada among descendents of Japanese and Chinese immigrants[13].
These findings suggest that genetic susceptibility for CD also exists among people of the Far East, where the real incidence of the disease is clearly underestimated because their dietary habits are different (more rice than wheat, in the staple diet), and possibly for the interest of doctors to recognize CD. These concerns pose important issues, which require further studies of CD in the different countries of this important and extensive area of the world.
CD is also very prevalent in people from the Middle East and these data are not surprising as they inhabit countries included in the “Fertile Crescent” such as Anatolia (South of Turkey), Lebanon, Syria, Palestine and Iraq, where some 10 000 years ago, some tribes changed from a nomadic lifestyle, to one of stable settlement, started the agriculture and probably propagated the spread of this disease throughout the world[14].
Thus, gluten intolerance appears to be a widespread public health problem and an increased level of awareness and clinical suspicion are needed to diagnose CD all over the world. Moreover, the GFD possess a big problem in developing countries, since commercial gluten-free products are not available, while in others they are very expensive and are difficult to acquire.
The clinical presentation of CD varies greatly, ranging from asymptomatic to severely malnourished patients. The most common clinical manifestations of CD include abdominal cramping pain with moderate to severe abdominal distension, frequently associated with relapsing or permanent dyspepsia, presence of gastro-esophageal reflux (GERD) and recurrent episodes of altered bowel habits (diarrhea and/or constipation), weight loss, bone disease, anemia and weakness.
While diarrhea was almost considered a persistent symptom, this is not the case in adults, and up to 50% of patients predominantly have constipation, which on many occasions becomes refractory to all types of therapy. It should be noted, that up to 30% of celiac patients have increased body mass index (BMI) and obvious obesity at diagnosis.
CD is sometimes divided into clinical subtypes. The terms “symptomatic or classic” apply to cases that meet the typical features described above. By contrast, in the “atypical forms” of the disease, the gastrointestinal symptoms may be absent or less pronounced, and in this case the extra-intestinal features predominate, such as chronic iron deficiency anemia, osteoporosis, short stature or failure to thrive, infertility and increased number of abortions.
Since atypical presentations are found more fre-quently in later decades, CD is now considered to be a multisystemic disorder, rather than a sole gastrointestinal process (Table 1).
| First degree relatives |
| Down´s and turner´s syndromes |
| IgA selective deficiency |
| Endocrine diseases |
| Type 1 diabetes mellitus |
| Autoimmune thyroid diseases |
| Alopecia areata |
| Neurologic diseases |
| Cerebellar ataxia |
| Epilepsy |
| Peripheral neuropathy |
| Multiple sclerosis |
| Liver diseases |
| Primary biliary cirrhosis |
| Autoimmune hepatitis |
| Autoimmune cholangitis |
| Idiopathic hypertransaminasemia |
| Rheumatologic diseases |
| Rheumatoid arthritis |
| Sjögren´s syndrome |
| Heart diseases |
| Idiopathic dilated cardiomyopathy |
| Autoimmune myocarditis |
| Cutaneous diseases |
| Dermatitis herpetiformis |
| Psoriasis |
| Vitiligo |
| Others |
| Iron-deficiency anemia Osteoporosis |
| Increased risk of fractures |
| Infertility |
| Amenorrhea |
| Dental enamel defects |
| Depression and anxiety |
| Chronic asthenia |
The triggers for CD are specific immunogenic peptides that are present only and exclusively in the dietary gluten proteins, from wheat and similar structural cereals such as rye and barley. These peptides are resistant to digestion by gastric and pancreatic enzymes and find their way into the lamina propria of the small bowel, presumably after some changes occur in the intercellular tight junctions with an increase in the intestinal permeability. One such peptide is a 33-amino acid sequence, which is a potent activator of specific T-cell lines from patients with CD[15].
The subsequent infiltration by CD4 (+) T lymphocytes into the lamina propria and CD8 (+) into the intestinal epithelium, are a hallmark of active CD. The recognition of HLA-bound gluten peptides by T cells, leads to their activation and clonal expansion of B cells that produce antibodies. Other cytokines released by activated CD4 T cells that involve the adaptive immune response, promote various inflammatory mechanisms and produce the intestinal lesion.
Less information is available on the activation and mode of action of intraepithelial T cells, which are mediated by the innate immune system. The expression of the interleukin-15 cytokine appears to play a central role in driving various processes that lead to the increased number of intraepithelial lymphocytes (IELs) as well as in the destruction process of the epithelial cells and the mucosal damage[16].
Tissue transglutaminase 2 (tTG), plays an important role in the immune response and is present in several tissues in the body. The cross-linking activity of tTG is involved in several functions, such as wound healing, formation of cell envelopes in apoptosis and stabilization of the extra-cellular matrix. In addition, this enzyme can deaminate glutamine residues. Glutamine-rich gluten peptides are, therefore, excellent substrates for tTG. The resulting deaminated and thus, negatively-charged peptides, have much higher affinity for the HLA-DQ2 and DQ8 molecules, and have a key step in the immune response in CD[17].
In summary, we must say that CD is a complex disorder that results from an interplay of several genetic, immunological and environmental factors, with many aspects for which the final pathogenetic mechanisms remains to be solved.
Among the serological tests needed to diagnose CD, the measurement of anti-gliadin IgA antibodies (AGA), has completely fallen into disuse and probably justifiably abandoned, as its sensitivity and specificity is very low (around 50%). In 1997, tTG was established by Dieterich et al to be the auto-antigen for anti-endomysial antibodies[18] and since then, is preferred for clinical use, because it show good sensitivity, greater than 90%, and a high specificity, around 95%, although it displays small variations between the different commercial kits employed[19].
The presence of these antibodies correlates with the degree of villous atrophy and various studies have clearly shown that the sensitivity of testing tTG is decreased in patients with normal duodenal biopsies or with mild histological changes[20].
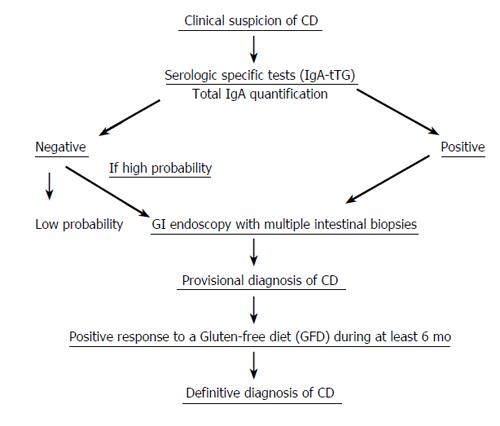
Measurement of tTG antibodies of the IgA isotype is usually determined in the clinical practice. Nevertheless, IgA deficiency occurs in 1.7%-2.6% of CD patients, which represents 10-15 times increase, over that in the general population. If IgA deficiency is found, measuring the IgG class tTG is recommended[21]. Diagnosis of CD based solely on serologic markers is not accepted and the identification of the characteristics changes at the duodenal mucosa is required before starting on a GFD.
In conclusion, in clinical practice, serologic tests for CD are frequently used to identify both symptomatic and asymptomatic at-risk individuals who require an intestinal biopsy examination to confirm the diagnosis.
The findings on a duodenal biopsy must be interpreted in detail by a pathologist experienced and interested in this condition and according to Marsh’s criteria (modified), which stratify this disease into four types or stages. Biopsy samples are usually collected during an upper GI endoscopy in the second portion of the duodenum close to the Vater ampulla; at least 4-6 well-oriented samples should be submitted, as the CD lesions are often irregular and patchily distributed[22].
CD may be diagnosed with a normal duodenal biopsy (Stage 0), with an increased percentage of intraepithelial lymphocytes (IELs) above 30% (Stage 1), the presence of crypt hyperplasia and inflammatory infiltrates in the lamina propria (Stage 2), and all of them with well-preserved villi. It is not until Stage 3, that villous atrophy develops, which is divided into three different categories: Mild (A), Moderate (B), and Total or Subtotal (C). Finally, Stage 4, or total hypoplasia of the mucosa, is now rarely seen.
Routine use of Marsh’s classification in the study of duodenal biopsies, is of great help, when recognizing occult, doubtful cases, and must always be used when performing a diagnostic upper endoscopy in patients belonging to so-called “at risk groups” regarding CD, including those with chronic diarrhea, refractory ferropenic anemia, or both[23].
Duodenal biopsy is still considered by most authors the “gold standard” in the diagnostic process, albeit its usefulness in the adult is still slightly controversial. If results of the histological study are negative but serologic tests are positive and CD is strongly suspected, the results of the biopsy should be reviewed with an expert gastrointestinal pathologist before additional biopsies are considered. In addition, if the histological exam yields equivocal results, it is useful to proceed with HLA typing. Although almost 40% of the general population has the HLA-DQ2 or HLA-DQ8 markers, nearly 90%-95% of CD patients have them[24].
The main diagnostic criteria still rest on duodenal biopsy findings while the patient is following a normal diet and whether significant clinical and analytical improvement is seen while the patient is on a strict GFD. Patients usually undergo hematological and biochemical tests along with the determination of serological CD markers (usually tTG-IgA class) by ELISA, when this disease is suspected. If serology results are negative, but clinical suspicion is high, intestinal biopsy should be performed. These biopsies must be oriented correctly in order to determine the degree of villous atrophy and to assess the presence and quantify the number of IELs, epithelial disarray, crypts hyperplasia and the degree of chronic inflammation present at the lamina propria.
A clear clinical improvement while the patient is following the GFD yields a definitive diagnosis. The serum antibodies generally disappear by 6 to 12 mo, although they are not necessarily a reliable indication of the mucosal response[25].
When patients do not present with the classic clinical symptoms of CD, a second biopsy that shows histologic improvement will confirm the diagnosis. An algorithm for a possible diagnosis of celiac disease, consisting of several consecutive steps, is shown (Figure 1).
For diagnostic confirmation in uncertain cases, several potential strategies may be agreed upon according to patient and family preferences. Amongst them, we shall quote the following: (a) repeat serology and laboratory tests periodically (b) perform a full family exploration, searching the presence of new potential cases (c) perform a new endoscopy with biopsy samples (less accepted) (d) try a GFD for 6 mo, monitoring the clinical response as well as the serologic and analytical changes. Gluten challenge is not considered necessary for diagnosis, except in patients for whom no initial diagnostic biopsy was done, or results of biopsy are unclear or uncharacteristic of CD. Patients should be informed that they may have a severe reaction with the gluten challenge.
The mainstay of treatment is a strict lifelong adherence to a GFD, in which the patient must avoid permanently all kinds of food products containing some wheat, rye, barley and oat. Commonly substituted grains, including rice and corn taken directly, or in the form of flour derived from them.
GFD is a very healthy and complete diet, very well balanced in all the immediate principles and rich enough in all kind of vitamins and minerals; it is the only type of treatment required for these patients. Compliance is a difficult task, at any age, because the wheat flour is present in a great part of foods or as an additive product. Patients whose disease does not respond to dietary treatment should undergo a systematic review[26,27].
The only gluten-free cereal is corn and is, therefore, allowed in the celiac patient’s diet whether raw or roasted, in salads, etc. Corn flour, if pure in its composition (100%) regarding preparation, processing and manufacture, may be used for bread, cookies, baby food and sweets, which may render a celiac patient’s diet more bearable.
Dairy products must be avoided when starting on a GFD, as secondary lactase deficiency is often associated with celiac disease. After 1-2 mo on a GFD, milk derivatives may be gradually reintroduced as long as the patient has no complaints following ingestion, since lactose intolerance is secondary to gluten, and usually regresses within three months with strict GFD adherence. On the other hand, dietary calcium and proteins are essential to correct existing mineral deficiencies, given the high prevalence of osteoporosis seen in celiac patients[28].
Beer must be completely avoided, even from alcohol-free brands, as its manufacture involves the fermentation of various gluten-containing cereals, including barley and rye. The same can be said of all kinds of whisky, obtained from malt distillation procedures. Approximately 70% of patients experience symptoms improvement after 2 wk on a GFD.
The rapidity and extent of histological regression are unpredictable, but there is invariably a delay against clinical improvement, which may not be apparent in repeated biopsies until after three to six months. While histological findings usually regress in children, one half of adults only achieve a partial histological resolution.
When there is severe iron-deficiency anemia, the administration of iron preparations through the intra-venous or intramuscular route is recommended for a few months (2 or 3), in order to shorten the recovery time. The two most important questions to answer are if the patient truly has CD and whether he/she is following a strict GFD. The evaluation requires a review of the original biopsies and a complete assessment by an expert dietician. Several associated conditions must be ruled out including the concomitant presence of pancreatic insufficiency, bacterial overgrowth, lymphocytic colitis and true refractory sprue with a clonal T-cell population[29,30].
Gluten sensitivity is relatively associated with several skin alterations but the most common presentation is in a form of chronic pruritic disease characterized by the presence of several symmetrical papulo-vesicular rash that evolves to crusting lesions broadly distributed over the body, but especially on the forearms, knees, buttocks, wrists and scalp. It is well known as dermatitis herpetiformis (DH) and the skin biopsy shows the characteristic lineal, granular deposits of IgA in the dermal papillae. This condition affects about 15%-25% of patients with CD and its presence is known as the “card presentation” of some CD patients[31].
A GFD is the treatment of choice, although at the start of the diet, drug therapy may be added, usually dapsone, to effectively and quickly resolve the itching and the rash. This drug suppresses the inflammation of the skin but obviously has no any influence on the intestinal abnormalities. The resolution of the cutaneous lesions may be slow, and some patients must wait 1-2 years after starting on a GFD, for the complete disappearance of DH. The intestinal biopsies show identical changes to CD, but predominate with mild lesions and a patchy distribution. tTG positivity is also similar, although at lower levels, possible reflecting a milder enteropathy. One study has shown the presence of antibodies exclusively against tTG-3 (also known as epidermal transglutaminase), a cytosolic enzyme involved in cell envelope formation during keratinocyte differentiation[32]. Although these findings remain to be confirmed, these may offer some clues to understanding the difference in clinical presentation between CD patients with, or without, associated skin lesions.
CD patients can exhibit some immune-mediated endocrine disorders alongside their clinical evolution, the most common being type 1 diabetes mellitus (T1DM) and thyroid disease. Each of these conditions affects 5%-10% of CD patients throughout their lives[33].
The prevalence of CD in the T1DM patients is substantially higher than expected in the general popu-lation. Approximately one half of subjects do not have gastrointestinal (GI) symptoms and the rest have only mild digestive disturbances. Indeed, many diabetic patients undergo endoscopy to investigate the frequent GI symptoms that afflict those with T1DM. It would require little extra effort or cost, to obtain duodenal biopsies, at least once to identify CD, and the biopsy result may explain the GI procedure has been done. It is not clear what impact that discovery has, if any, on diabetic control or complications, although GI symptoms seem to improve on a GFD. The GFD influence on the control or management of thyroid disease is limited at best, and additional studies are clearly needed to reach firm conclusions[34,35].
One interesting study performed in Italian children found a high incidence of autoimmune thyroid disease in 90 of 343 (26.2%) patients with CD (62 on a GFD) and in 20 (10%) of the control subjects (P = 0.001). Fifty-four (15.7%) patients with CD and autoimmune markers had normal thyroid function (euthyroidism) as did 12 (6.0%) of the control subjects. Hypothyroidism was observed in 28 (8.1%) patients with CD and in 7 (3.5%) of the control subjects. Hyperthyroidism was diagnosed in four patients with CD and in none of the control subjects with autoimmune markers. An abnormal echographic pattern was seen in 37 patients with CD (16.8%) and only in one (1.6%) of the control subjects (P = 0.002). The high frequency of autoimmune thyroid disease found among patients with CD, even those on a GFD, may justify a thyroid status assessment at diagnosis and at follow-up evaluation of children and adults with CD[36].
Diet iron is absorbed by the proximal small intestine, the site of greatest damage in CD. It is not surprising therefore, that iron-deficiency anemia is a common finding in newly diagnosed CD. It also usually resolves with the institution of a GFD[37].
Several studies from Europe and North America have suggested that iron-deficiency anemia may be the sole manifestation of CD in the absence of diarrhea. This association may be especially high in those unresponsive to oral iron therapy[38].
Iron deficiency is common in the general population. If it occurs in young women, it is often ascribed to excess menstrual loss, an empiric therapy with oral iron supplementation is started. However, older patients or those with anemia that is refractory to treatment are often investigated further. Similarly, the persistence of anemia after menopause may be an important clue that leads to the detection of CD[39].
Indeed, female patients have undergone hysterectomies to treat the iron deficiency that persisted until the correct diagnosis was made. Anemia is rarely sought or diagnosed in children; in fact, hemoglobin is not routinely measured in children. Nevertheless, iron-deficiency anemia is a very common illness in primary care and often does not spur investigation in the younger patients. The prevalence of CD in patients referred to GI endoscopy for investigation of iron-deficiency anemia varies from 3% to 12%[40,41].
Clinicians should consider CD as a possible, although not common, cause of unexplained anemia, and gastroenterologists should biopsy the duodenum when an endoscopy is performed in patients with iron-deficiency anemia, even if biopsies are not specifically required.
Low bone mass, is common in patients with newly diagnosed CD. The mechanism for this effect may be due to malabsorption of vitamin D and calcium and decreased intake of calcium, because of lactose intolerance. Other factors such as sex, malnutrition and physical activity, also contribute to the risk of low bone density in CD[42,43].
Decrease in bone mineral density (BMD) associated with CD responds to a GFD, with a gradual restoration to normal, over two years. The earlier in life that treatment is started, the better is the response[44]. A limited number of screening studies for CD, among patients with low bone mass (LBM), have been performed in Europe. CD was found in 3.4% in adults with LBM[45].
However, a carefully performed Canadian study in predominantly postmenopausal women has not identified an increased prevalence of CD. One likely explanation is the way the low bone mineral density is defined. Individuals with BMD more than 2.5 standard deviations below the sex-specific peak bone mass are presumed to have osteoporosis. Therefore, it seems that screening those patients with simple postmenopausal osteoporosis, as defined by World Health Organization (WHO) criteria, is unhelpful[46].
Recently, the risk of fractures due to osteoporosis has become a major subject of interest. CD has been associated with an increase in fracture risk. What effect silent, undiagnosed CD, has on lifelong risk fracture risk, is not well known[47].
A large population-based cohort study performed in Great Britain showed that the overall hazard ratio in CD patients for any fracture was 1.30 and 1.90 for hip fracture[48].
The prevalence of hypertransaminasemia (HT) in children is increased in CD. In one study a total of 114 consecutive pediatric CD patients were studied (60% with classical and 40% with atypical forms). The authors found HT in 32% of patients, at the time of diagnosis. In five patients, it was the only manifestation of CD (4.3%). Patients with HT were younger (2.9 ± 0.4 year) than patients with normal aminotransferases (5.1 ± 0.5 year) (P = 0.007). A higher percentage of patients with classical CD tends to have abnormal aminotransferases (73%; 95% CI = 65-81%) than do patients with atypical CD (27%; 95% CI = 19-35%) (P = 0.068). A younger age was significantly associated with HT (P = 0.039; OR = 0.8; 95% CI = 0.71-0.99). The aminotransferases normalized with a GFD in all 35 patients, who were followed-up for an average time of one year[49].
A significant percentage of adult patients with non-alcoholic fatty liver disease (NAFLD) have no metabolic risk factors and may be related with the concomitant presence of CD. Bardella et al found in a series of 59 patients that tissue transglutaminase antibodies were positive in six (10%) patients and the anti-endomysium in two (3.4%); only two (3.4%), positive for both anti-endomysium and anti-transglutaminase, demonstrated CD on histological findings. After 6 mo of a GFD, liver enzymes normalised[50].
Mild liver abnormalities are common in adult patients with celiac disease and usually resolve with a GFD. Four patients with untreated celiac disease and severe liver disease are described by Kaukinnen et al of Finland. Further, the occurrence of celiac disease was studied in 185 adults with previous liver transplantation using tTG and endomysium antibodies (EMA) testing. Of the four patients with severe liver disease and celiac disease, one had congenital liver fibrosis, one had massive hepatic steatosis and two had progressive hepatitis without any apparent origin. Three patients were even remitted for consideration for liver transplantation. Hepatic dysfunction reversed in all cases when a gluten-free diet was adopted. In the transplantation group, 8 patients (4.3%) had celiac disease. Six cases were detected before the operation: 3 had primary biliary cirrhosis, one had autoimmune hepatitis, one had primary sclerosing cholangitis and one had congenital liver fibrosis. Only one patient had maintained a long-term, strict gluten-free diet. The serological screening found 2 cases of celiac disease, one with autoimmune hepatitis and the other with secondary sclerosing cholangitis. The possible presence of celiac disease should be investigated in patients with severe liver disease. Dietary treatment may prevent progression to hepatic failure, even in cases in which liver transplantation is considered[51].
Among the most common neurology problems associated with CD are peripheral neuropathy, cerebellar ataxia, epilepsy, multiple sclerosis and migraine. In a recent study of 26 patients with CD, 31% had abnormalities in neurophysiologic studies, compared with 4% of controls with reflux disease[52].
Nutritional factors have been suspected in association with neurological defects but are rarely found and their correction does not seem to influence in the prognosis. Some reports show certain neurologic symptoms that respond to a GFD, especially if it is started in the first few months after their appearance[53].
It is now evident that the link between CD and neurologic disorders results, in part, from common genetic background, most importantly, the HLA region on chromosome 6, and other markers. In addition to genetic predisposition, immunologic factors probably also play a role[54,55].
One way that this may occur is by antibody or T-cell cross-reactivity, a mechanism that is suspected of triggering the immune response in some autoimmune diseases. Alternatively, it may result from the involvement of additional autoantigens through epitope spreading.
The GFD effect on epilepsy control has been variable. In most patients, some beneficial effects have been reported such as better seizure control and a decrease in dosing of anti-epileptic medications, but without achieving a complete resolution of seizures with the diet alone[56].
Depression and other psychiatric symptoms have been reported as common complications of CD, occurring in about one third of patients. Common symptoms include apathy, excessive anxiety and irritability. All of them clearly improve after few months of adherence to a GFD[57].
The incidence of certain types of cancer is increased among patients with CD. These include non-Hodgkin’s lymphomas (NHL) at any site, enteropathy-associated T-cell lymphoma (EATL) (a rare, high-grade T-cell non-Hodgkin’s lymphoma of the small intestine), for which the outlook is poor, small intestine adenocarcinoma and esophageal and oropharyngeal carcinomas[58-60]. In one large cohort study performed on 4732 CD patients compared with 23 620 matched controls, the authors found in CD, an increased RR factor for mortality of 1.39 with a 95% CI (1.13-1.51) and for malignancy of 1.29, CI-95% (1.06-1.55)[61]. The mechanisms responsible for the development of malignancies in CD patients are not known. The following explanations have been suggested: increased intestinal permeability of environmental carcinogens, chronic inflammation, chronic antigen stimulation, release of pro-inflammatory cytokines, immune surveillance problems and nutritional deficiencies caused by the disease or the GFD[62].
CD is clearly associated with a definite increase in the risk of developing cancer, especially EATL and other gastrointestinal cancers that are partially responsible for the overall increased mortality reported in these patients. However, the magnitude of the overall risk for NHL is much lower than previously thought, with a relative risk most probably ranging between 2 and 4[63,64]. Strict adherence to a GFD seems to protect against the development of some types of cancer[65,66].
Several issues may be of help, as listed in the following decalogue: (1) Clinicians must have a better understanding of CD, have good knowledge of this common disease (1%-2% in the general population), and consider it in the differential diagnosis of multiple gastrointestinal and extraintestinal conditions. (2) It is very important to do a careful clinical history dating back to childhood, looking for a relation of periodic complaints to food ingestion, exploring the family’s history of CD and searching for associated disease such as recurrent rhinitis, pharyngo-amigdalitis, otitis, sinusitis, asthma, and other immuno-allergic conditions. (3) To perform a systematic screening of CD in high risk patients such as those presenting with iron deficiency anemia, especially if it is refractory, and taking routine duodenal biopsies during upper gastrointestinal endoscopies in these patients. (4) In the presence of sustained hypertransaminasemia, in the absence of a history of liver disease, and when viral markers are negative, screen for CD, which is associated with the former conditions in around 10% of cases. (5) If endocrine disturbances are present, such as hypo- or hyperthyroidism, or positive anti-thyroidal antibodies with normal function are present, and also in the presence of associated type 1 diabetes mellitus, consider a potential case of CD. (6) Remember that screening serology, particularly the measurement of anti-transglutaminase antibodies, can sometimes be negative in adult patients. (7) Duodenal biopsy may be normal or show only minimal changes. An experienced pathologist deeply interested in the diagnosis of CD, and routinely using Marsh’s classification in his or her reports, is needed. (8) Bear in mind that being DQ2 (+) is a necessary but insufficient condition, and that increasingly more cases are being diagnosed in DQ2 (-) individuals. (9) In doubtful cases, the introduction of a GFD may be suggested for a minimum of six months, after which the clinical and laboratory response is observed (“ex-iuvantibus” diagnosis). (10) Finally, the Gastroenterology Units in the tertiary hospitals should include monographic “Small Bowel Sections” for the routine study of these patients, and should be fitted with modern functional techniques and endoscopic procedures for their study, such as video-capsule and double-balloon enteroscopy.
| 1. | James MW, Scott BB. Coeliac disease: the cause of the various associated disorders? Eur J Gastroenterol Hepatol. 2001;13:1119-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Catassi C, Ratsch IM, Fabiani E, Rossini M, Bordicchia F, Candela F, Coppa GV, Giorgi PL. Coeliac disease in the year 2000: exploring the iceberg. Lancet. 1994;343:200-203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 456] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 594] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Lopez-Vazquez A, Rodrigo L, Fuentes D, Riestra S, Bousoño C, Garcia-Fernandez S, Martinez-Borra J, Gonzalez S, Lopez-Larrea C. MHC class I chain related gene A (MICA) modulates the development of coeliac disease in patients with the high risk heterodimer DQA1*0501/DQB1*0201. Gut. 2002;50:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Greco L, Romino R, Coto I, Di Cosmo N, Percopo S, Maglio M, Paparo F, Gasperi V, Limongelli MG, Cotichini R. The first large population based twin study of coeliac disease. Gut. 2002;50:624-628. [RCA] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 6. | Collin P, Kaukinen K, Välimäki M, Salmi J. Endocrinological disorders and celiac disease. Endocr Rev. 2002;23:464-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | O'Leary C, Walsh CH, Wieneke P, O'Regan P, Buckley B, O'Halloran DJ, Ferriss JB, Quigley EM, Annis P, Shanahan F. Coeliac disease and autoimmune Addison's disease: a clinical pitfall. QJM. 2002;95:79-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lo W, Sano K, Lebwohl B, Diamond B, Green PH. Changing presentation of adult celiac disease. Dig Dis Sci. 2003;48:395-398. [RCA] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Ferguson A, Arranz E, O'Mahony S. Clinical and pathological spectrum of coeliac disease--active, silent, latent, potential. Gut. 1993;34:150-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 265] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1150] [Article Influence: 50.0] [Reference Citation Analysis (2)] |
| 11. | Yachha SK, Misra S, Malik AK, Nagi B, Mehta S. Spectrum of malabsorption syndrome in north Indian children. Indian J Gastroenterol. 1993;12:120-125. [PubMed] |
| 12. | Bhatnagar S, Gupta SD, Mathur M, Phillips AD, Kumar R, Knutton S, Unsworth DJ, Lock RJ, Natchu UC, Mukhopadhyaya S. Celiac disease with mild to moderate histologic changes is a common cause of chronic diarrhea in Indian children. J Pediatr Gastroenterol Nutr. 2005;41:204-209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Freeman HJ. Biopsy-defined adult celiac disease in Asian-Canadians. Cand J Gastr. 2003;17:433-436. |
| 14. | Rostami K, Malekzadeh R, Shahbazkhani B, Akbari MR, Catassi C. Coeliac disease in Middle Eastern countries: a challenge for the evolutionary history of this complex disorder? Dig Liver Dis. 2004;36:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1112] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 16. | Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, Auricchio S, Picard J, Osman M, Quaratino S, Londei M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30-37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Molberg O, McAdam SN, Sollid LM. Role of tissue transglutaminase in celiac disease. J Pediatr Gastroenterol Nutr. 2000;30:232-240. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797-801. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1352] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 19. | Fernández E, Riestra S, Rodrigo L, Blanco C, López-Vázquez A, Fuentes D, Moreno M, López-Larrea C. Comparison of six human anti-transglutaminase ELISA-tests in the diagnosis of celiac disease in the Saharawi population. World J Gastroenterol. 2005;11:3762-3766. [PubMed] |
| 20. | Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol. 2003;36:219-221. [RCA] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and "Club del Tenue" Working Groups on Coeliac Disease. Gut. 1998;42:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992;102:330-354. [PubMed] |
| 23. | Riestra S, Domínguez F, Fernández-Ruiz E, García-Riesco E, Nieto R, Fernández E, Rodrigo L. Usefulness of duodenal biopsy during routine upper gastrointestinal endoscopy for diagnosis of celiac disease. World J Gastroenterol. 2006;12:5028-5032. [PubMed] |
| 24. | Dewar D, Pereira SP, Ciclitira PJ. The pathogenesis of coeliac disease. Int J Biochem Cell Biol. 2004;36:17-24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Sategna-Guidetti C, Grosso S, Bruno M, Grosso SB. Reliability of immunologic markers of celiac sprue in the assessment of mucosal recovery after gluten withdrawal. J Clin Gastroenterol. 1996;23:101-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Culliford AN, Green PH. Refractory sprue. Curr Gastroenterol Rep. 2003;5:373-378. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ojetti V, Nucera G, Migneco A, Gabrielli M, Lauritano C, Danese S, Zocco MA, Nista EC, Cammarota G, De Lorenzo A. High prevalence of celiac disease in patients with lactose intolerance. Digestion. 2005;71:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830-1938. [RCA] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 464] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 31. | Reunala TL. Dermatitis herpetiformis. Clin Dermatol. 2001;19:728-736. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368:377-396. [RCA] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 780] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 33. | Ivarsson A, Persson LA, Nystrom L, Ascher H, Cavell B, Danielsson L, Dannaeus A, Lindberg T, Lindquist B, Stenhammar L. Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000;89:165-171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Kolho KL, Farkkila MA, Savilahti E. Undiagnosed coeliac disease is common in Finnish adults. Scand J Gastroenterol. 1998;33:1280-1283. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Kaukinen K, Salmi J, Lahtela J, Siljamäki-Ojansuu U, Koivisto AM, Oksa H, Collin P. No effect of gluten-free diet on the metabolic control of type 1 diabetes in patients with diabetes and celiac disease. Retrospective and controlled prospective survey. Diabetes Care. 1999;22:1747-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Ansaldi N, Palmas T, Corrias A, Barbato M, D'Altiglia MR, Campanozzi A, Baldassarre M, Rea F, Pluvio R, Bonamico M. Autoimmune thyroid disease and celiac disease in children. J Pediatr Gastroenterol Nutr. 2003;37:63-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Bodé S, Gudmand-Høyer E. Symptoms and haematologic features in consecutive adult coeliac patients. Scand J Gastroenterol. 1996;31:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Corazza GR, Valentini RA, Andreani ML, D'Anchino M, Leva MT, Ginaldi L, De Feudis L, Quaglino D, Gasbarrini G. Subclinical coeliac disease is a frequent cause of iron-deficiency anaemia. Scand J Gastroenterol. 1995;30:153-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Ransford RA, Hayes M, Palmer M, Hall MJ. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. J Clin Gastroenterol. 2002;35:228-233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Ackerman Z, Eliakim R, Stalnikowicz R, Rachmilewitz D. Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anemia. Am J Gastroenterol. 1996;91:2099-2102. [PubMed] |
| 41. | Grisolano SW, Oxentenko AS, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The usefulness of routine small bowel biopsies in evaluation of iron deficiency anemia. J Clin Gastroenterol. 2004;38:756-760. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Delco F, El-Serag HB, Sonnenberg A. Celiac sprue among US military veterans: associated disorders and clinical manifestations. Dig Dis Sci. 1999;44:966-972. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Bernstein CN, Leslie WD. The pathophysiology of bone disease in gastrointestinal disease. Eur J Gastroenterol Hepatol. 2003;15:857-864. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Mora S, Barera G, Ricotti A, Weber G, Bianchi C, Chiumello G. Reversal of low bone density with a gluten-free diet in children and adolescents with celiac disease. Am J Clin Nutr. 1998;67:477-481. [PubMed] |
| 45. | Mazure R, Vazquez H, Gonzalez D, Mautalen C, Pedreira S, Boerr L, Bai JC. Bone mineral affection in asymptomatic adult patients with celiac disease. Am J Gastroenterol. 1994;89:2130-2134. [PubMed] |
| 46. | Mather KJ, Meddings JB, Beck PL, Scott RB, Hanley DA. Prevalence of IgA-antiendomysial antibody in asymptomatic low bone mineral density. Am J Gastroenterol. 2001;96:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Fisher AA, Davis MW, Budge MM. Should we screen adults with osteoporotic fractures for coeliac disease? Gut. 2004;53:154-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | West J, Logan RF, Card TR, Smith C, Hubbard R. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003;125:429-436. [RCA] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Farre C, Esteve M, Curcoy A, Cabré E, Arranz E, Amat LL, Garcia-Tornel S. Hypertransaminasemia in pediatric celiac disease patients and its prevalence as a diagnostic clue. Am J Gastroenterol. 2002;97:3176-3181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Bardella MT, Valenti L, Pagliari C, Peracchi M, Farè M, Fracanzani AL, Fargion S. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Kaukinen K, Halme L, Collin P, Färkkilä M, Mäki M, Vehmanen P, Partanen J, Höckerstedt K. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 52. | Luostarinen L, Himanen SL, Luostarinen M, Collin P, Pirttila T. Neuromuscular and sensory disturbances in patients with well treated coeliac disease. J Neurol Neurosurg Psychiatry. 2003;74:490-494. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Hadjivassiliou M, Davies-Jones GA, Sanders DS, Grunewald RA. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74:1221-1224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 937] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 55. | Fdez-Morera JL, Tunon A, Rodriguez-Rodero S, Rodrigo L, Martinez-Borra J, Gonzalez S, Lopez-Vazquez A, Lahoz CH, Lopez-Larrea C. Clinical behavior of multiple sclerosis is modulated by the MHC class I-chain-related gene A. Tissue Antigens. 2006;67:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Gobbi G, Bouquet F, Greco L, Lambertini A, Tassinari CA, Ventura A, Zaniboni MG. Coeliac disease, epilepsy, and cerebral calcifications. The Italian Working Group on Coeliac Disease and Epilepsy. Lancet. 1992;340:439-443. [RCA] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Ciacci C, Iavarone A, Mazzacca G, De Rosa A. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol. 1998;33:247-250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Askling J, Linet M, Gridley G, Halstensen TS, Ekstrom K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428-1435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (2)] |
| 59. | Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 61. | West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Catassi C, Fabiani E, Corrao G, Barbato M, De Renzo A, Carella AM, Gabrielli A, Leoni P, Carroccio A, Baldassarre M. Risk of non-Hodgkin lymphoma in celiac disease. JAMA. 2002;287:1413-1419. [RCA] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 63. | Schweizer JJ, Oren A, Mearin ML. Cancer in children with celiac disease: a survey of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;33:97-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Howdle PD, Jalal PK, Holmes GK, Houlston RS. Primary small-bowel malignancy in the UK and its association with coeliac disease. QJM. 2003;96:345-353. [RCA] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Loftus CG, Loftus EV. Cancer risk in celiac disease. Gastroenterology. 2004;123:1726-1729. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Holmes GK, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989;30:333-338. [RCA] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 602] [Article Influence: 16.3] [Reference Citation Analysis (14)] |
S- Editor Liu Y L- Editor Lakatos PL E- Editor Liu WF