Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6531
Revised: August 12, 2006
Accepted: September 11, 2006
Published online: October 28, 2006
AIM: To address the possibility that insulin-like growth factor (IGF)-II is a growth factor and its signaling pathway so as to develop a molecular therapy for hepatoblastoma.
METHODS: Huh-6 and HepG2, human hepatoblastoma cell lines, were used. IGF-II was added to the medium deprived of serum. Western blot analysis was performed to clarify the expression of IGF-I receptor (IGF-IR). Inhibitors of IGF-IR (picropodophyllin, PPP), phosphatidyl-inositol (PI) 3-kinase (LY294002 and Wortmannin), or mitogen-activated protein (MAP) kinase (PD98059) were added to unveil the signaling pathway of IGF-II. Cells were analyzed morphologically with hematoxylin-eosin staining to reveal the mechanism of suppression of cell proliferation.
RESULTS: IGF-II stimulated cells proliferated to 2.7 (269% ± 76%) (mean ± SD) (Huh-6) and 2.1 (211% ± 85%) times (HepG2). IGF-IR was expressed in Huh-6 and HepG2. PPP suppressed the cell number to 44% ± 11% (Huh-6) and 39% ± 5% (HepG2). LY294002 and Wortmannin suppressed the cell number to 30% ± 5% (Huh-6), 44% ± 0.4% (HepG2), 49% ± 1.0% (Huh-6) and 46% ± 1.1% (HepG2), respectively. PD98059 suppressed the cell number to 33% ± 11% for HepG2 but not for Huh-6. When cell proliferation was prohibited, many Huh-6 and HepG2 cells were dead with pyknotic or fragmented nuclei, suggesting apoptosis.
CONCLUSION: IGF-II was shown to be a growth factor of hepatoblastoma via IGF-I receptor and PI3 kinase which were good candidates for target of molecular therapy.
- Citation: Tomizawa M, Saisho H. Signaling pathway of insulin-like growth factor-II as a target of molecular therapy for hepatoblastoma. World J Gastroenterol 2006; 12(40): 6531-6535
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6531
Hepatoblastoma (HBL) arises in the liver of infants younger than 3 years of age[1]. The growth factor and its signaling pathway could be revealed so as to develop a novel molecular therapy for HBL.
Insulin-like growth factor (IGF)-II is a hormone that plays an important role in fetal growth and development. IGF-II is abundantly expressed in fetus, with its concentration decreasing after birth[2]. Interestingly, IGF-II is detected at high levels in the serum of HBL patients[3]. Moreover, the expression levels of IGF-II in tumor tissues are higher than those of surrounding non-tumor tissues in surgical specimens from HBL patients due to biallelic expression of the gene by loss of the methylated status of the promoter[4]. These facts indicate that IGF-II is deeply involved in the carcinogenesis and progression of HBL.
IGF-II binds to insulin receptor (IR), IGF-I receptor (IGF-IR), and IGF-II receptor[5]. With the binding of IGF-II, IR mediates glucose metabolism, such as insulin. IGF-IIR mediates the degradation of IGF-II, acting as a tumor-suppressor gene. It was expected that IGF-IR mediated the stimulation of proliferation by IGF-II. Indeed, antibody to IGF-IR successfully suppressed the proliferation of HepG2[6]. Once IGF-II binds to IGF-IR, the receptor autophosphorylates and activates phosphatidyl-inositol (PI) 3-kinase or mitogen-activated protein (MAP) kinase. Further down-stream pathways modulate the gene expression to perform the role of IGF-II.
However, it is not clear whether IGF-II promotes the cell proliferation of HBL, or the signaling pathway of IGF-II to mediate the stimulation of cell proliferation.
Here, we tried to address the possibility of IGF-II in HBL as a growth factor. We also tried to clarify its signaling pathway with inhibitors to signaling pathways, pursuing a potential application for a molecular therapy.
Huh-6 and HepG2 hepatoblastoma cell lines, were purchased from RIKEN Cell Bank (Tsukuba, Japan) and cultured in Dulbecco’s Minimum Essential Medium (Sigma, St. Louis, MO) supplemented with 100 g/L fetal bovine serum (FBS) (Trace Scientific, Melbourne, Australia) in 50 mL/L at 37°C in a humidified chamber. Both cell lines are IGF-IR positive[7]. For hematoxylin-eosin (H&E) staining, cells were spread onto chamber slides.
Freshly thawed cells were seeded onto 10 cm dishes (Asahi Techno Glass, Funabashi, Japan). When they reached sub-confluence, they were trypsinized, harvested, and spread onto 96-well flat-bottom plates with (Asahi Techno Glass) at a density of 1000 cells per well. Following 24 h of culture under DMEM with 100 g/L FBS, medium was changed to DMEM without FBS to quench the FBS effects. After 24 h of culture under DMEM without FBS, IGF-II (Wako Pure Chemicals, Osaka, Japan) was added to the medium. Seventy-two hours later, 3- (4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay was performed according to the manufacturer’s instructions (Promega Corporation, Tokyo, Japan). MTS is bioreduced by cells into a colored formazan product that reduces absorbance at 490 nm. The absorbance was analyzed with a multiple plate reader at a wavelength of 490 nm with a BIO-RAD Model 550 microplate reader (Bio-RAD, Hercules, CA). When LY290042 (Wako Pure Chemicals), PD98059 (Wako Pure Chemicals), Wortmannin (Wako Pure Chemicals), and picropodophyllin (PPP) (Wako Pure Chemicals) were used, each was added to the medium 30 min prior to the addition of IGF-II.
Twenty microgram of protein isolated from cultured cells was subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis, and transferred to a nylon filter. Primary antibodies were polyclonal rabbit anti-IGF-IR antibody (Cell Signaling Technology, Danvers, MA) and mouse monoclonal anti-Tubulin-α antibody (Lab Vision, Fremont, CA). Second antibodies were horseradish peroxidase (HRP)-linked anti-rabbit antibody (Amersham Bioscience, Tokyo, Japan) and HRP-linked anti-mouse antibody (Amersham Bioscience). Dilutions were 1:500 for primary antibodies, and 1:1000 for second antibodies. The filter was reprobed with anti-Tubulin-α antibody. The specific antigen-antibody complexes were visualized by enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp, Piscataway, NJ).
Cell proliferation, demonstrated by MTS assay, was analyzed statistically by one-factor analysis of variance. Statistical analysis was performed with JMP5.0J (SAS Institute Japan, Tokyo, Japan). P < 0.05 was accepted as statistically significant.
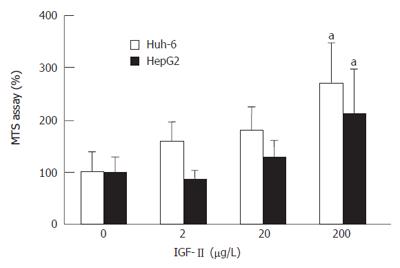
Huh-6 and HepG2 proliferated as the concentration of IGF-II increased (Figure 1). When IGF-II was 200 μg/L, cell proliferation of Huh-6 and HepG2 were 2.7 times (269% ± 76%) (mean ± SD) and 2.1 times (211% ± 85%) higher than those at 0 μg/L of IGF-II, respectively (P < 0.05, n = 3).
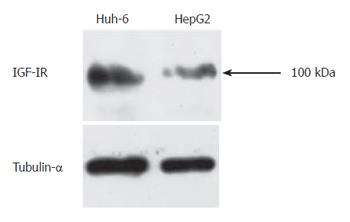
Western blot analysis was performed to analyze expression of IGF-IR in Huh-6 and HepG2 since IGF-IR mediates proliferation activity of IGF-II. Protein was isolated from Huh-6 and HepG2 72 h after stimulation with IGF-II (200 μg/L). IGF-IR was expressed in Huh-6 and HepG2 (Figure 2). Tubulin-α was expressed to confirm that equal amount of protein was loaded (Figure 2).
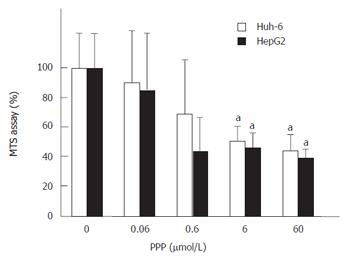
PPP, a selective inhibitor of IGF-IR, was used to show that IGF-IR mediated the signal of IGF-II (Figure 3). PPP at 60 μmol/L suppressed the cell number of Huh-6 and HepG2 stimulated with 200 μg/L of IGF-II to 44% ± 10% and 39% ± 5%, respectively (P < 0.05, n = 3).
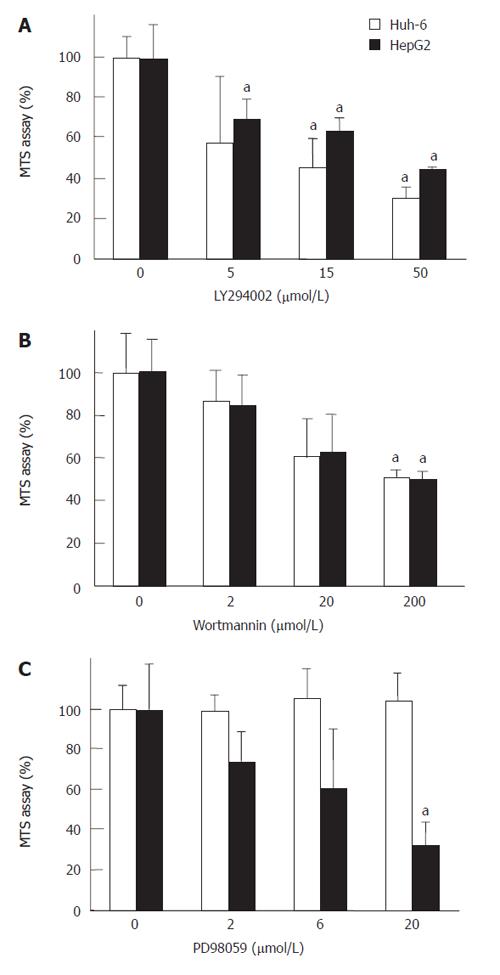
LY294002 and Wortannin, specific inhibitors of PI3 kinase, were used to reveal that PI3 kinase mediated the signal of IGF-II (Figure 4). LY294002 at 50 μmol/L and Wortmannin at 200 μmol/L suppressed the cell numbers of Huh-6 and HepG2 stimulated with 200 μg/L of IGF-II to 30% ± 5% and 44% ± 0.4% (Figure 4A) (P < 0.05, n = 3), and 49% ± 1.0% and 46% ± 1.1% (Figure 4B) (P < 0.05, n = 3), respectively. PD98059, a specific inhibitor of MAP kinase, was used to clarify whether MAP kinase mediated the signal of IGF-II. PD98059 did not suppress the proliferation of Huh-6 even at 20 μmol/L, while it suppressed that of HepG2 to 33% ± 11%, which was statistically significant (P < 0.05, n = 3) (Figure 4C).
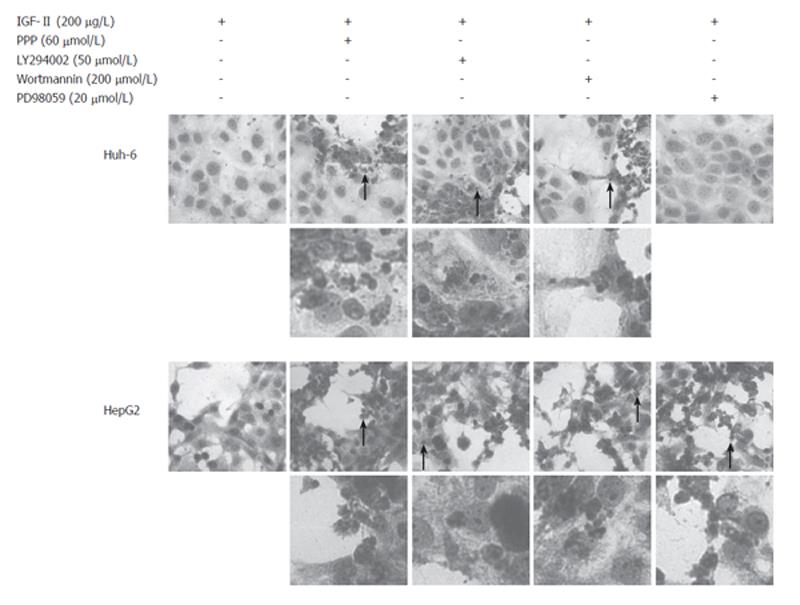
Cultured cells were HE stained to analyze the morphological change 72 h after addition of inhibitors, Huh-6 with PPP, LY294002, and Wortmannin but not with PD98059 while HepG2 with PPP, LY294002, Wortmannin, and PD98059 (Figure 5). Most of the dead cells had pyknotic or fragmented nuclei, indicating apoptosis.
The existence of a growth factor in HBL has not been confirmed. It is reported that the expression of IGF-II is elevated in tumor tissues and serum of HBL patients, but its exact role is not clear in terms of carcinogenesis[8]. In this study, we demonstrated that IGF-II stimulated the proliferation of HBL cell lines. A previous report showed that IGF-II does not stimulate the proliferation of HepG2[6]. They added IGF-II at a concentration of 200 μg/L as well as fetal bovine serum. We added 200 μg/L of IGF-II to the medium deprived of serum. Serum stimulates the proliferation of HepG2 to obscure the effect of IGF-II. Moreover, we analyzed Huh-6, another human hepatoblastoma cell line, and revealed that IGF-II stimulated the proliferation of Huh-6[9]. Our data clearly demonstrated that IGF-II stimulated the proliferation of hepatoblastoma cell lines. Interestingly, HepG2 produces IGF-II and antisense oligonucleotides of IGF-II suppress the proliferation. It may be safe to conclude that IGF-II acts as a growth factor for HBL by autocrine action[6,10].
The previous report suggested that IGF-II stimulates proliferation via IGF-IR[6]. Our data clearly showed that IGF-IR was expressed in Huh-6 and HepG2. Since IGF-IR mediates proliferation activity of IGF-II, it was expected that an inhibitor of IGF-IR suppressed proliferation[5]. NVP-AEW541, a tyrosine kinase inhibitor, suppresses the proliferation of HepG2[7]. We used the commercially available PPP, another tyrosine kinase inhibitor, and it successfully suppressed the proliferation of Huh-6 and HepG2[11]. Our data on HepG2 was consistent with that of the previous report, and thus was strong evidence that IGF-II mediated the signaling pathway of IGF-II. Since IGF-IR is not involved in glucose metabolism, an inhibitor of IGF-II would be a good candidate for molecular therapy for hepatoblastoma.
PI3 kinase and MAP kinase are the main downstream molecules of IGF-IR[5]. It was reported that the proliferation of HepG2 cells is suppressed by LY294002 and PD98059[12], and our results confirmed this. Our results also showed that LY294002 suppressed the proliferation of Huh-6, but PD98059 did not. One may speculate that the stimulation of IGF-II was transmitted via PI3 kinase and MAP kinase in HepG2, but only via PI3 kinase in Huh-6. Wortmannin, another inhibitor of PI3 kinase, suppressed the proliferation of Huh-6 and HepG2 stimulated by IGF-II, confirming the results of LY294002.
MTS assay measured cell viability, representing cell proliferation. The mechanism of suppression of cell proliferation, however, was not known. To unveil the mechanism, morphological analysis was performed with HE staining. Many of Huh-6 cells were dead with PPP, LY294002, and Wortmannin while HepG2 with PPP, LY294002, Wortmannin, and PD98059. Nuclei of dead cells were pyknotic or fragmented, indicating apoptosis. It is reported that DNA ladder formation is observed when HepG2 is treated with LY294002, Wortmannin, or PD98059[5]. It was suggested that Huh-6 and HepG2 were dead due to apoptosis when IGF-I signaling pathway was inhibited. It may be concluded that PI3 kinase would be a better target than MAP kinase for the molecular therapy of hepatoblastoma.
| 1. | Ishak KG, Glunz PR. Hepatoblastoma and hepatocarcinoma in infancy and childhood. Report of 47 cases. Cancer. 1967;20:396-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Brown AL, Graham DE, Nissley SP, Hill DJ, Strain AJ, Rechler MM. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986;261:13144-13150. [PubMed] |
| 3. | Dong ZZ, Yao DF, Yao DB, Wu XH, Wu W, Qiu LW, Jiang DR, Zhu JH, Meng XY. Expression and alteration of insulin-like growth factor II-messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655-4660. [PubMed] |
| 4. | Poirier K, Chalas C, Tissier F, Couvert P, Mallet V, Carrié A, Marchio A, Sarli D, Gicquel C, Chaussade S. Loss of parental-specific methylation at the IGF2 locus in human hepatocellular carcinoma. J Pathol. 2003;201:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 798] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | Lund P, Schubert D, Niketeghad F, Schirmacher P. Autocrine inhibition of chemotherapy response in human liver tumor cells by insulin-like growth factor-II. Cancer Lett. 2004;206:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Höpfner M, Huether A, Sutter AP, Baradari V, Schuppan D, Scherübl H. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol. 2006;71:1435-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Gray SG, Eriksson T, Ekström C, Holm S, von Schweinitz D, Kogner P, Sandstedt B, Pietsch T, Ekström TJ. Altered expression of members of the IGF-axis in hepatoblastomas. Br J Cancer. 2000;82:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Tokiwa T, Doi I, Sato J. Preparation of single cell suspensions from hepatoma cells in culture. Acta Med Okayama. 1975;29:147-150. [PubMed] |
| 10. | Lin SB, Hsieh SH, Hsu HL, Lai MY, Kan LS, Au LC. Antisense oligodeoxynucleotides of IGF-II selectively inhibit growth of human hepatoma cells overproducing IGF-II. J Biochem (Tokyo). 1997;122:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene. 2004;23:7854-7862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Mitsui H, Takuwa N, Maruyama T, Maekawa H, Hirayama M, Sawatari T, Hashimoto N, Takuwa Y, Kimura S. The MEK1-ERK map kinase pathway and the PI 3-kinase-Akt pathway independently mediate anti-apoptotic signals in HepG2 liver cancer cells. Int J Cancer. 2001;92:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Ma JY E- Editor Ma WH