Published online Oct 21, 2006. doi: 10.3748/wjg.v12.i39.6339
Revised: May 28, 2006
Accepted: June 15, 2006
Published online: October 21, 2006
AIM: To evaluate sample adequacy, safety, and needle passes of a new biopsy needle device compared to the Quick-Core biopsy needle for transjugular liver biopsy in patients affected by liver disease.
METHODS: Thirty consecutive liver-disease patients who had major coagulation abnormalities and/or relevant ascites underwent transjugular liver biopsy using either a new needle device (18 patients) or the Quick-Core biopsy needle (12 patients). The length of the specimens was measured before fixation. A pathologist reviewed the histological slides for sample adequacy and pathologic diagnoses. The two methods’ specimen adequacy and complication rates were assessed.
RESULTS: Liver biopsies were technically successful in all 30 (100%) patients, with diagnostic histological core specimens obtained in 30 of 30 (100%) patients, for an overall success rate of 100%. With the new device, 18 specimens were obtained, with an average of 1.1 passes per patient. Using the Quick-Core biopsy needle, 12 specimens were obtained, with an average of 1.8 passes per patient. Specimen length was significantly longer with the new needle device than with the Quick-Core biopsy needle (P < 0.05). The biopsy tissue was not fragmented in any of the specimens with the new aspiration needle device, but tissue was fragmented in 3 of 12 (25.0%) specimens obtained using the Quick-Core biopsy needle. Complications included cardiac arrhythmia in 3 (10.0%) patients, and transient abdominal pain in 4 (13.3%) patients. There were no cases of subcapsular hematoma, hemoperitoneum, or sepsis, and there was no death secondary to the procedure. In particular, no early or delayed major procedure-related complications were observed in any patient.
CONCLUSION: Transjugular liver biopsy is a safe and effective procedure, and there was significant difference in the adequacy of the specimens obtained using the new needle device compared to the Quick-Core biopsy needle. Using the new biopsy needle device, the specimens showed no tissue fragmentation and no increment in major procedure-related complications was observed.
- Citation: Ishikawa T, Kamimura H, Tsuchiya A, Togashi T, Watanabe K, Seki KI, Ohta H, Yoshida T, Ishihara N, Kamimura T. Comparison of a new aspiration needle device and the Quick-Core biopsy needle for transjugular liver biopsy. World J Gastroenterol 2006; 12(39): 6339-6342
- URL: https://www.wjgnet.com/1007-9327/full/v12/i39/6339.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i39.6339
To diagnose the cause and stage of liver disease, tran-sjugular liver biopsy is an effective way to obtain a liver specimen in patients with a contraindication to percutaneous biopsy, such as coagulopathy or ascites[1-3]. Several different kinds of biopsy needles have been designed, including the modified Ross needle (Cook, Bloomington, Indiana) placed through a 9-F curved sheath[4], and a side-cutting needle, such as the Quick-Core needle (Cook Medical, Bloomington, IN. USA) placed through a 7-F curved sheath with a guiding metal cannula inside the sheath[5]. A widely used, single-use biopsy needle, the Quick-Core system uses an 18-, 19-, or 20-gauge cutting needle with an automated spring-fired mechanism to obtain tissue. The popularity of the Quick-Core system is its relative ease of use and high diagnostic yield. The success rate for transjugular liver biopsy is high, but sometimes tissue samples inadequate to secure a diagnosis are obtained by this device. The present report describes the results obtained by a new biopsy device of our own design for transjugular liver biopsy.
In our hospital, we use two types of needle: a new aspiration needle device of our own design (supplied by Hakko Co Ltd, Nagano, Japan) and the Quick-Core biopsy needle (Cook Medical). To our knowledge, there have been only a few reports comparing the safety and adequacy of the different types of needles. The purpose of this study was to evaluate and compare the safety, complications, and tissue sample adequacy, including the diagnostic yield, of transjugular liver biopsy using the two different needles.
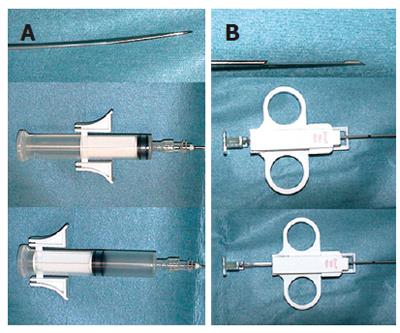
Between April 2002 and April 2006, 30 patients had a transjugular liver biopsy at Saiseikai Niigata Second Hospital. There were 15 men and 15 women, aged 22-75 years, with an average age of 46.57 years. All cases had a contraindication for percutaneous liver biopsy due to coagulation abnormalities and/or massive ascites. Coagulation abnormalities were defined as an INR of 1.4 or greater, and a platelet count less than 30 000/cm3. After sedation and local anesthesia, a transjugular liver biopsy was performed in 18 patients using an 18-gauge new aspiration needle device (Figure 1A), and in 12 patients using the 18-gauge Quick-Core Biopsy Needle (Figure 1B) System. With the new device, histological core liver specimens were obtained by manually aspirating through the inner cannula. All patients gave their informed consent after being fully informed of the nature of the study. The study was approved by the Ethical Committee of our hospital and was conducted in accordance with the principles of the Declaration of Helsinki. Patients were randomly allocated using a computer generated randomization code to undergo transjugular liver biopsy with the new device or with the automated Quick-Core device. The randomization results were securely kept in sealed opaque envelopes.
A 9-F, 45-cm vascular sheath (Cook Medical), was placed over a 0.035-inch guide wire into the right internal jugular vein according to the Seldinger technique, and then a 7-F multipurpose catheter was advanced under fluoroscopic control into the right, middle, or left hepatic vein. Subsequently, contrast venography was done to determine the actual position of the catheter before biopsy. With the new device, the aspiration biopsy needle was advanced within the catheter to its tip, and then the specimen was obtained by aspiration while further pushing it into the parenchyma in an antero-inferior direction. With the 18-guage, 60-cm-long, side-cutting automated biopsy device (Quick-Core needle) the needle was positioned in the same direction, and one or several passes were done to obtain an adequate sample. Fluoroscopy and ECG monitoring were used throughout the procedure in both groups.
After the biopsy procedure, contrast medium was injected through the catheter to rule out capsular perforation. The retrieved specimens were mounted longitudinally on dry paper, and their lengths were measured before formalin fixation. An adequate amount of liver tissue was obtained and placed in formalin for pathologic evaluation. The samples were considered fragmented if the largest core sample was less than 6 mm in length. The procedure was considered to be technically successful if a liver tissue sample was obtained. If the pathologist was able to determine the histological diagnosis based on the sample provided, the procedure was considered diagnostic. One experienced hepatobiliary pathologist was not informed about the technique. She evaluated the histological slides to obtain a pathologic diagnosis and to determine the adequacy of the submitted specimens. Complications during the procedure were recorded, and patients’ clinical records were reviewed for any delayed complications. The Mann-Whitney U-test was used to statistically analyze any differences between the two groups.
Table 1 presents the indications for transjugular biopsy in our hospital (Table 1). In 70.0% of the patients in this study, coagulation disorders, thrombocytopenia, or ascites were the indications for transjugular biopsy.
| (1) Increased risk of liver bleeding: 16 cases (53.3%) |
| Severe coagulation disorders (14 cases) including: |
| -Thrombocytopenia: 1 cases (due to hypersplenism or hematological disorders) |
| -Fulminant hepatitis (Severe acute hepatitis): 13 cases |
| Miscellaneous disorders (2 cases) including: |
| -Hematological diseases (DIC): 2 cases |
| (2) Measurement of hepatic pressure gradient: 4 cases (13.3%) |
| (3) Massive ascites: 5 cases (16.6%) |
| (4) Miscellaneous: suspicion of amyloidosis or peliosis, obesity, failure of percutaneous liver biopsy: 5 cases (16.6%) |
The clinical features of the patients included in the study and the indications for biopsy were similar between the two groups (Table 2).
| Clinical feature | New device(n = 18) | Quick-core biopsy needle(n = 12 ) |
| Sex (M/F) | 9/9 | 6/6 |
| Mean age ± SD (range) (yr) | 46.6 ± 15.4 (22-72) | 49.4 ± 16.3 (27-75) |
| Indications | ||
| Increased risk of bleeding: | 9 | 7 |
| Measurement of hepatic pressure gradient: | 3 | 1 |
| Massive ascites: | 3 | 2 |
| Miscellaneous: | 3 | 2 |
| (1) Liver cirrhosis: 9 cases (30.0%) |
| Alcohol: 5 cases AIH: 3 cases IPH: 1 case |
| (2) Viral hepatitis: 14 cases (46.6%) |
| Fulminant: Massive necrosis: 4 cases, Submassive necrosis: 9 cases, |
| Chronic: 1 case |
| (3) Unknown Origin: 2 cases (6.6%) |
| Focal necrosis: 2 cases |
| (4) Tumoral diseases: 2 cases (6.6%) |
| Metastasis: 1 case, Lymphoma: 1 case |
| (5) Others: 3 cases (9.9%) |
| Intrahepatic cholestassis: 1 case, NASH s/o: 1 case, |
| Amyloidosis s/o: 1 case |
The histological diagnoses included: cirrhosis (9 patients, 30.0%), viral hepatitis (14 patients, 46.6%), focal necrosis (2 patients, 6.6%), tumoral diseases (2 patients, 6.6%), and others (3 patients, 9.9%).
| New device(n = 18) | Quick-core biopsy needle(n = 12) | |
| Tissue Length (mm) | 15.3 ± 5.1 (10-21) | 6.8 ± 2.1 (3-10) |
| Number of portal triads | 6.5 ± 1.2 | 3.5 ± 0.6 |
| Number of fragments | 0/18 (0%) | 3/12 (25%) |
| Number of passes | 1.1 ± 0.2 | 1.8 ± 0.8 |
| Minutes (min) | 28.5 ± 7.9 | 45.1 ± 9.9 |
Transjugular biopsy was technically successful in 30 of 30 (100%) cases. A total of 30 specimens (18 for the new needle device and 12 for the Quick-Core biopsy needle) were obtained, with an average of 1.4 needle passes per patient (1.1 for the new needle device and 1.8 for the Quick-Core biopsy needle). However, the tissue was fragmented in 3 (25%) of 12 specimens obtained using the Quick-Core biopsy needle. In contrast, there was no tissue fragmentation in any of the 18 specimens obtained using the new aspiration needle device. Furthermore, all 12 specimens obtained using the Quick-Core biopsy needle were shorter than 10 mm, probably because of insufficient parenchymal puncture. Although sample fragmentation was present in 3 (25%) cases, all 12 procedures were diagnostic (100%).
The lengths of the specimens obtained using the Quick-Core biopsy needle were significantly shorter (3-10 mm, mean = 6.8 mm) than the lengths of the specimens obtained using the new needle device (10-21 mm, mean = 15.3 mm, P < 0.05). All of the specimens obtained using the new biopsy needle device were adequate for making a diagnosis (100%). Transjugular liver biopsy with the new aspiration device was more effective than with the Quick-Core biopsy needle with respect to obtaining an accurate diagnosis. The mean procedure time was stastically significant shorter using the new needle device (28.5 ± 7.9 min) than with the Quick-Core biopsy needle (45.1 ± 9.9 min).
| New device(n = 18) | Quick-core biopsy needle(n = 12 ) | |
| Cardiac arrhythmia | ||
| (Supraventricular tachycardia) | 1 | 2 |
| Transient fever | 0 | 1 |
| Transient abdominal pain | 1 | 3 |
There were no early or late complications, such as capsular perforation, hemoperitoneum, or puncture site hematoma with either device.
Three minor complications were reported in 8 (26.6%) patients; there were no major complications. Three patients developed supraventricular tachycardia during the procedure, which was believed to have been a pre-existing condition. The patients’ supraventricular tachycardia responded to medical therapy with conversion to normal sinus rhythm within 24 h. Other minor complications included transient abdominal pain (n = 4) and transient fever (n = 1) that spontaneously resolved before the procedure was completed. The complication rate with the new device was significantly lower than with the Quick-Core needle.
Percutaneous liver biopsy allows one to obtain adequate tissue samples with minimal injury to the liver. However, the procedure is contraindicated in patients with an increased risk of hemorrhage, such as those with marked coagulopathy, thrombocytopenia, or ascites[6,7]. In 1964, Dotter reported successfully biopsying the livers of several dogs using a transvenous catheter[8]. Subsequently, Hanafee and Weiner first described the technique of transjugular catheterization of the hepatic veins in human subjects[9]. Three years later, the first human transjugular liver biopsies were performed in a few patients by the same authors[10].
In 1973, Rosch and colleagues reported the first major clinical series of transjugular liver biopsy with a description of 44 biopsies[11].
Today, transjugular liver biopsy is an established alter-native procedure for patients who have a contraindication for percutaneous biopsy. The factors that affect the quality of the specimen obtained are: type of needle, operator skill, and texture of the liver parenchyma. The selection of a needle for doing liver biopsy is based on needle safety, ease of manipulation, and reliability in retrieving specimens of an adequate length that are not fragmented. The new needle device and the Quick-Core biopsy needle use different mechanisms to obtain tissue samples. The new device uses an aspiration (suction) mechanism, while the Quick-Core device uses a swift side-cutting motion (Figure 1). The Quick-Core biopsy needle is part of a semi-automated 18-gauge Trucut needle biopsy gun device, composed of a cutting cannula and an inner stylet with a 20-mm specimen notch. Papatheodoridis et al[12] found no difference in the adequacy of the specimens obtained by the Quick-Core system and the Colapinto needle, but they did not specify the core size.
We report our experience of 18 transjugular liver biopsy procedures using the 18-gauge new needle device system. The technical success rate of the biopsy was 100%. In our study, there were no cases of intraperitoneal or subcapsular bleeding; this may have been due to the use of routine venography after the biopsy passes.
DeHoyos et al[13] reported a mean of 2.4 ± 1.0 passes, Papatheodoridis et al[12] reported a mean of 1.8 ± 1.0 passes, Choh et al[14] reported a mean of 2.8 passes, and Gonzalez-Tutor et al[15] reported a mean of 3.1 passes. A larger number of needle passes contributes to a higher incidence of complications, most notably perforation of the liver capsule. In our study, 30 specimens (18 with the new needle device and 12 with the Quick-Core biopsy needle) were obtained, with an average of 1.4 needle passes per patient (1.1 for the new needle device and 1.8 for the Quick-Core biopsy needle). The number of passes was significantly lower with the new needle device than with the Quick-Core system.
The specimens obtained were significantly longer with the new needle device (10-21 mm, mean = 15.3 mm) than with the Quick-Core biopsy needle (3-10 mm, mean = 6.8 mm, P < 0.05).
The transjugular liver biopsy specimens obtained using the new needle device had a high diagnostic yield comparable to those obtained using the Quick-Core biopsy system. A critical disadvantage of the Quick-Core system needle is frequent tissue fragmentation. Previously, transjugular liver biopsy done using conventional aspiration techniques has been shown to yield insufficient tissue samples. It is known that a suction or aspiration biopsy of a cirrhotic liver is likely to result in tissue fragmentation. Using the Quick-Core biopsy needle, the tissue was fragmented in 3 (25%) of 12 specimens, while there was no tissue fragmentation in any of the 18 specimens obtained using the new aspiration needle device.
Therefore, the new needle device system would appear to be superior to the Quick-Core system transseptal needle for obtaining pathologically adequate specimens. The length of all specimens obtained with the Quick-Core needle was less than 10 mm, due primarily to insufficient puncture of liver parenchyma. In our study, no procedure-related problems occurred with our new needle device system. There were no cases of intraperitoneal hemorrhage, even without the insertion of plugging material into the biopsy tract.
In conclusion, transjugular liver biopsy is a safe and effective procedure in high-risk liver-disease patients for whom percutaneous biopsy is contra-indicated. With the new biopsy needle device, larger specimens are usually obtained without tissue fragmentation. In summary, transjugular liver biopsy using the new needle device is safe and has a high diagnostic yield in patients with advanced liver disease.
| 1. | Velt PM, Choy OG, Shimkin PM, Link RJ. Transjugular liver biopsy in high-risk patients with hepatic disease. Radiology. 1984;153:91-93. [PubMed] |
| 2. | Lipchik EO, Cohen EB, Mewissen MW. Transvenous liver biopsy in critically ill patients: adequacy of tissue samples. Radiology. 1991;181:497-499. [PubMed] |
| 3. | Gamble P, Colapinto RF, Stronell RD, Colman JC, Blendis L. Transjugular liver biopsy: a review of 461 biopsies. Radiology. 1985;157:589-593. [PubMed] |
| 4. | Corr P, Beningfield SJ, Davey N. Transjugular liver biopsy: a review of 200 biopsies. Clin Radiol. 1992;45:238-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Little AF, Zajko AB, Orons PD. Transjugular liver biopsy: a prospective study in 43 patients with the Quick-Core biopsy needle. J Vasc Interv Radiol. 1996;7:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 807] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 8. | Dotter CT. Catheter biopsy. Experimental technic for transvenous liver biopsy. Radiology. 1964;82:312-314. [PubMed] |
| 9. | Hanafee W, Weiner M. Transjugular percutaneous cholangiography. Radiology. 1967;88:35-39. [PubMed] |
| 10. | Weiner M, Hanafee WN. A review of transjugular cholangiography. Radiol Clin North Am. 1970;8:53-68. [PubMed] |
| 11. | Rösch J, Lakin PC, Antonovic R, Dotter CT. Transjugular approach to liver biopsy and transhepatic cholangiography. N Engl J Med. 1973;289:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Papatheodoridis GV, Patch D, Watkinson A, Tibballs J, Burroughs AK. Transjugular liver biopsy in the 1990s: a 2-year audit. Aliment Pharmacol Ther. 1999;13:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | De Hoyos A, Loredo ML, Martínez-Ríos MA, Gil MR, Kuri J, Cárdenas M. Transjugular liver biopsy in 52 patients with an automated Trucut-type needle. Dig Dis Sci. 1999;44:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Choh J, Dolmatch B, Safadi R, Long P, Geisinger M, Lammert G, Dempsey J. Transjugular core liver biopsy with a 19-gauge spring-loaded cutting needle. Cardiovasc Intervent Radiol. 1998;21:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Gonzalez-Tutor A, García-Valtuille R, Cerezal L, Bustamante M. Transjugular biopsy of the liver with an automated device. Acta Radiol. 1998;39:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Chiarioni G E- Editor Bai SH