Published online Sep 28, 2006. doi: 10.3748/wjg.v12.i36.5757
Revised: July 5, 2006
Accepted: July 11, 2006
Published online: September 28, 2006
H pylori is now accepted as the cause of gastritis and gastritis-associated diseases, such as duodenal ulcer, gastric ulcer, gastric carcinoma, and gastric MALT lymphoma. The natural history of H pylori gastritis includes inflammation progressing from the antrum into the adjacent corpus resulting in an atrophic front of advancing injury leading to a reduction in acid secretion and eventual loss of parietal cells and development of atrophy. Sub-typing intestinal metaplasia has no clinical value to the patient, the pathologist, or the endoscopist. The pattern, extent, and severity of atrophy, with or without intestinal metaplasia, is a far more important predictor than is intestinal metaplasia subtype. The challenge remains to identify a reliable marker that relates to pre-malignant potential.
- Citation: El-Zimaity HM. Gastric atrophy, diagnosing and staging. World J Gastroenterol 2006; 12(36): 5757-5762
- URL: https://www.wjgnet.com/1007-9327/full/v12/i36/5757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i36.5757
H pylori is now accepted as the cause of gastritis and gastritis-associated diseases, such as duodenal ulcer, gastric ulcer, gastric carcinoma, and gastric MALT lymphoma. Overall, two rules are clear: (1) the pattern of gastritis is the major determinant of disease outcome[1,2], and (2) countries with a high prevalence of gastric cancer and gastric ulcer, such as Japan or Peru, have a low incidence of duodenal ulcer[3]. Duodenal ulcer is typically associated with antral predominant gastritis, little or no atrophy and normal or increased acid secretion[4-7]. Gastric ulcer and intestinal gastric cancer are typically associated with extensive gastritis, widespread intestinal metaplasia and hypo- or achlorhydria[3,4,8,9]. However, both rules can be broken[9,10]: (1) wide spread intestinal cancer has been documented in the corpus of Korean duodenal ulcer patients, and (2) both diseases (duodenal ulcer and gastric cancer) are frequent diagnoses in dyspeptic Korean patients[9,10]. One of the keys to this apparent paradox is a person’s natural acid secretory status.
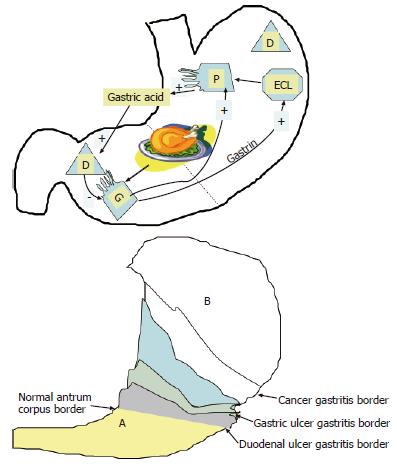
Although H pylori are found throughout the stomach, in the early stages of disease, H pylori-associated inflammation is often mild, superficial, or even absent in the gastric corpus[10,11]. The natural history of H pylori gastritis is for the inflammation to progress from the antrum into the adjacent corpus resulting in an atrophic front of advancing injury, leading to a reduction in acid secretion and eventually loss of parietal cells and development of atrophy[10,12,13]. This progression is not inevitable. In the general population it progresses at a rate of 1%-2% per year[12]. The rate of progression of gastritis differs among different countries, different regions of the same country and among different H pylori-related diseases[14,15]. Overall, the incidence of gastric cancer is highest in countries and regions with a high incidence of early development of atrophic corpus gastritis[12,16-19] (Figure 1). In contrast, in duodenal ulcer patients, gastritis tends to stay largely confined to the antrum and either does not advance, or spreads very slowly, to involve the gastric corpus[12,20,21]. Atrophic pangastritis with hypochlorhydria is rare or develops sufficiently late in life that the risk of gastric cancer for the population of patients with duodenal ulcer remains low.
The rate of progression of H pylori gastritis progression depends on the acid milieu. Thus, H pylori corpus gastritis is accelerated in clinical scenarios associated with low acid secretion, such as chronic therapy with proton pump inhibitors, which are widely used in gastro-esophageal reflux disease[8,22-37]. Omeprazole therapy is associated with a reduction in bacterial load, both in the antrum and in the corpus, and a tendency for antral histology to improve and corpus gastritis to either not change or worsen. With omperazole therapy, not only does the corpus mucosa fail to show histologic improvement, but there is a significant progression of the inflammatory reaction deeper within the pit involving the proliferative zone[38].
A person’s natural acid secretory status thus appears to determine whether they will develop duodenal or gastric ulcer disease[39,40] with the acid secretory status appearing to affect both the distribution and severity of H pylori-related gastritis. There is some evidence that some cases of duodenal ulcer disease may “burn out” and this has been postulated to be due to the extension of gastritis into the corpus, thus reducing acid secretion to the point where it is no longer possible to sustain an active duodenal ulcer[41]. One possibility is that with continued inflammation, antral atrophy may lead to a sufficient destruction of gastrin producing cells[42] to produce a fall in acid secretion[43,44], which would allow the development of corpus gastritis. In most duodenal ulcer cases, gastritis extends slowly or not at all giving the impression of localization to the antrum[45]. Thus, antral predominant gastritis may in some instances represent an earlier stage of atrophic pangastritis such that these patterns actually represent two ends of the spectrum of “H pylori infection” rather than mutually exclusive diseases[10,46,47] (Figure 1).
The rate of progression from gastritis to atrophy varies in different geographic regions related to other environmental factors. While diet is probably the most important factor that reduces acid secretion, other factors such as childhood infections may be very important[10,14,48,49]. The rate of development and the proportion of the population with atrophic gastritis is a critical determinant for the risk of gastric cancer in that population[14,15]. The apparent higher prevalence of concomitant duodenal ulcer and gastric cancer in Korea[46] and the presence of atrophic gastritis with intestinal metaplasia in the corpus of Korean duodenal ulcer patients[10] suggest that in Korea the rate of expansion of the atrophic front is more rapid than in patients in other geographic areas.
This review only covers the histopathological diagnosis and staging of gastric atrophy; serologic measures are not addressed. The natural history of H pylori gastritis is to go through a cascade of events that involves non-atrophic gastritis, atrophic gastritis, and finally dysplasia[50-52].Atrophy begins at the fundic- or B-boundary line (defined as a margin between the corpus, with complete fundic gland mucosa, and the antrum)[18,53,54] as a sheet of pseudo-pyloric metaplasia with islands of intestinal metaplasia[10,13,55] and shifts proximally such that the antrum appears to expand replacing fundic gland mucosa with advancing atrophic gastritis[18,20,53,56]. Corpus atrophy progresses proximally to variably sized regions of the adjacent greater curve, proximal half of the lesser curve, and neighboring anterior and posterior walls of the corpus[13,18,53,57]. We will address intestinal metaplasia and pseudo-pyloric metaplasia separately.
Because the development of gastric carcinoma is a slow and unpredictable process, and intestinal metaplasia is an easily recognizable marker for atrophy, investigators have suggested that sub-typing intestinal metaplasia using high-iron diamine staining might identify subgroups of patients with different risk potential. Intestinal metaplasia sub-typed as III is often considered as a precursor lesion for the intestinal form of gastric cancer[58-61]. In practice, areas of intestinal metaplasia (or a certain sub-type) are generally small and can easily be missed at follow-up[62]. Sampling error is likely the critical factor responsible for the fact that an approximately equal number of studies have suggested that intestinal metaplasia regresses or does not regress after treatment of H pylori infection[62-70]. Prior studies suggesting an association of type III intestinal metaplasia with the development of gastric cancer[59-61,71] did not take into account the higher prevalence of incomplete intestinal metaplasia (type III) in the gastric antrum[13,72,73]. In addition, while type III intestinal metaplasia is present in all specimens with intestinal type gastric carcinoma, it can easily be missed in biopsy as it can be present in very small areas[13].
A small percentage of cancer patients can show complete replacement of the antrum mucosa with intestinal metaplasia and have normal appearing oxyntic mucosa[13]. It is unknown if these individuals had normal or reduced acid secretion. Continued inflammation with antral atrophy could possibly lead to sufficient destruction of gastrin producing cells[45], which can result in a fall in acid secretion[74,75]. Alternatively, contiguous sheets of intestinal metaplasia may be unstable epithelium especially upon exposure to carcinogens.
Overall, it is apparent that it is not currently possible to make recommendations or prognoses based on either a single or multiple biopsies showing sulphomucin in areas with intestinal metaplasia[62,72,76,77]. All data suggest that the extent of mucosal atrophy within a region of the stomach may have a more important relation with the intestinal type of gastric cancer than the presence or type of intestinal metaplasia. While intestinal metaplasia is a form of atrophy that is easy for pathologists to recognize, it is also important to determine whether intestinal metaplasia is present as an isolated patch within non-atrophic mucosa or amidst an atrophic lawn[10,13].
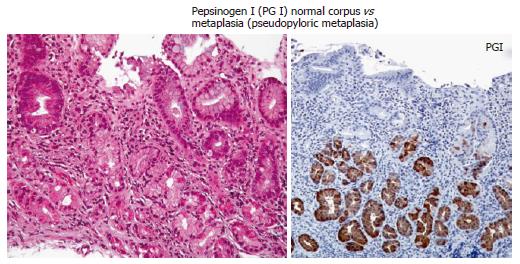
The normal oxyntic mucosa has straight glands composed of tightly packed chief cells, parietal cells, endocrine cells, and mucus cells with a higher ratio of glands to foveola than the antrum. With continuous inflammation, and progressive atrophy, there is a progressive loss of parietal cells. Eventually, the oxyntic mucosa glandular compartment can resemble antral/pyloric glands on H&E exam (pseudopyloric metaplasia). The diagnosis of pseudopyloric metaplasia can be facilitated by using pepsinogen I immunostain (e.g. anti-pepsinogen I from Biogenesis Kingston, NH). Pepsinogen I (PG I) is localized in chief cells, mucous-neck cells and transitional mucous-neck/chief cells of the human fundic mucosa[78]; it is not localized in antral gland cells (Figure 2). Pepsinogen II, on the other hand is localized in chief cells, mucous neck cells, and antral gland cells (Table 1). Pseudo-pyloric metaplasia is identified by the presence of mucosa that is phenotypically antrum, stains positive for pepsinogen I, and is anatomically in a region where corpus would be expected[13,55].
| PepsinogenI (PGI) | Pepsinogen II (PG II) | |
| Chief cells | Positive | Positive |
| Mucous neck cells | Positive | Positive |
| Antral gland cells | Negative | Positive |
Pseudopyloric metaplasia has been described as early as 1959[79] in benign gastric ulcers proximal to the normal border zone (antrum-corpus junction). In fact, prior to the rediscovery of H pylori, a proximally advancing atrophic front with pseudopyloric metaplasia was considered part of the normal aging process[18,56]. Following the rediscovery of H pylori, a positive association has been demonstrated between the presence of mucous glands in corpus biopsies (pseudo-pyloric or mucous metaplasia) and the age of H pylori infected patients. This association was more prevalent in Korea where gastric carcinoma is common[10]. The pattern of atrophy in the form of pseudo-pyloric metaplasia is considered regenerative in nature[80,81] and has been observed in experimental models[82], as well as in gastric remnants following distal gastrectomy with gastroenteric anastomosis[83]. In fact, routine screening for gastric cancer in asymptomatic patients with gastric remnants often reveals pseudo-pyloric metaplasia in oxyntic type mucosa.
In staging corpus atrophy, it is important to remember four rules: (1) atrophy begins at the border line (antrum-corpus border); (2) atrophy replaces fundic gland mucosa with both pseudopyloric metaplasia and/or intestinal metaplasia[13,53,84]; (3) the atrophic border extends proximally more rapidly up the lesser curve than the greater curvature such that locations high on the greater curvature are among the last to manifest atrophy[13,53,79,84]; and (4) the presence of a higher density of mucosa mononuclear cells that infiltrate deep into the lamina propria is a predictor for the presence of gastric atrophy[38,85].
In early stages of atrophic gastritis, observed in children[55], the location of the antral-corpus border would be expected to be nearer to the normal anatomic border[10]. As such, the identification of atrophy requires biopsies be taken close to the normal antrum corpus junction[55]. In contrast, the atrophic front (atrophic border) is expected to be more proximal in patients in developing countries, in countries with a high incidence of gastric carcinoma (Figure 1), and within particular groups in developed countries that have a higher incidence of gastric carcinoma, including the socially and economically disadvantaged[86], with the atrophic border advancing more proximally with age[10]. To note, the cardia is not only a high yield zone for H pylori[11], but also both intestinal metaplasia and pseudo pyloric metaplasia have been identified in the cardia of children with early atrophic gastritis[55].
The Sydney system and Updated Sydney system[87] were primarily designed to provide standardization for reports of gastric biopsies. The Sydney system[88] recommended a minimum of two biopsies from the respective gastric compartments to be taken from the anterior and posterior wall. In 1994, the Sydney system for the classification and grading of gastritis was updated. The recommendation was unchanged regarding the need for a minimum of two biopsies from the respective gastric compartments but the location was changed from the anterior and posterior walls to the greater and lesser curves of the stomach[87]. In both instances the sites were chosen arbitrarily. Though the Sydney biopsy sites have proven to provide reliable identification of H pylori infection[44,89,90], the sites recommended by the Sydney system can only identify corpus atrophy when it is extensive[10,62,90,91]. The recommended biopsy sites for research studies designed to identify the presence, pattern, or changes in atrophic gastritis over time or following a therapeutic intervention must be carefully selected to ensure that they encompass the advancing atrophic border. The number and sites chosen will therefore depend on the average degree and severity of atrophic gastritis expected in the population[13,55]. Use of a standardized reporting system, such as the updated Sydney system, is useful for biopsy specimens particularly as it promotes the use of a visual analog scale to score mucosal findings. For research we suggest the use of a 6 point scale[92] as it provides finer gradation than the 4 point scale used for reporting clinical specimens[87].
In summary, to increase our likelihood of identifying corpus atrophy, when present, special emphasis should be placed on: (1) targeting biopsy sites to encompass the likely sites of the advancing atrophic border and the cardia[55], (2) consistently including intestinal metaplasia and pseudopyloric metaplasia in our evaluation of corpus biopsies for atrophy[13,87], and lastly, (3) raising our suspicion for corpus atrophy in biopsies with a higher density of mucosa mononuclear cells that infiltrate deep into the lamina propria[38,85].
| 1. | Stemmermann GN, Hayashi T. Intestinal metaplasia of the gastric mucosa: a gross and microscopic study of its distribution in various disease states. J Natl Cancer Inst. 1968;41:627-634. [PubMed] |
| 2. | Lambert R. Chronic gastritis. A critical study of the progressive atrophy of the gastric mucosa. Digestion. 1972;7:83-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Burstein M, Monge E, León-Barúa R, Lozano R, Berendson R, Gilman RH, Legua H, Rodriguez C. Low peptic ulcer and high gastric cancer prevalence in a developing country with a high prevalence of infection by Helicobacter pylori. J Clin Gastroenterol. 1991;13:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Graham DY. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hepatol. 1991;6:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991;6:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615-625. [PubMed] |
| 8. | Meining A, Stolte M, Hatz R, Lehn N, Miehlke S, Morgner A, Bayerdörffer E. Differing degree and distribution of gastritis in Helicobacter pylori-associated diseases. Virchows Arch. 1997;431:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Malaty HM, Kim JG, El-Zimaity HM, Graham DY. High prevalence of duodenal ulcer and gastric cancer in dyspeptic patients in Korea. Scand J Gastroenterol. 1997;32:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | El-Zimaity HMT O, Kim JG, Akamatsu T, Gürer IE, Simjee AE, Graham DY. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol. 2001;96:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Siurala M, Sipponen P, Kekki M. Chronic gastritis: dynamic and clinical aspects. Scand J Gastroenterol Suppl. 1985;109:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | El-Zimaity HM, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35 Suppl 12:90-97. [PubMed] |
| 16. | Siurala M, Salmi HJ. Long-term follow-up of subjects with superficial gastritis or a normal gastric mucosa. Scand J Gastroenterol. 1971;6:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Villako K, Kekki M, Tamm A, Savisaar E. Development and progression of chronic gastritis in the antrum and body mucosa: results of long-term follow-up examinations. Ann Clin Res. 1986;18:121-123. [PubMed] |
| 18. | Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology. 1972;63:584-592. [PubMed] |
| 19. | Tatsuta M, Iishi H, Okuda S. Location of peptic ulcers in relation to antral and fundal gastritis by chromoendoscopic follow-up examinations. Dig Dis Sci. 1986;31:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Tarpila S, Kekki M, Samloff IM, Sipponen P, Siurala M. Morphology and dynamics of the gastric mucosa in duodenal ulcer patients and their first-degree relatives. Hepatogastroenterology. 1983;30:198-201. [PubMed] |
| 21. | Siurala M. Gastritis, its fate and sequelae. Ann Clin Res. 1981;13:111-113. [PubMed] |
| 22. | Lamberts R, Creutzfeldt W, Strüber HG, Brunner G, Solcia E. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Gastroenterology. 1993;104:1356-1370. [PubMed] |
| 23. | Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 495] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Meining A, Kiel G, Stolte M. Changes in Helicobacter pylori-induced gastritis in the antrum and corpus during and after 12 months of treatment with ranitidine and lansoprazole in patients with duodenal ulcer disease. Aliment Pharmacol Ther. 1998;12:735-740. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Stolte M, Bethke B. Elimination of Helicobacter pylori under treatment with omeprazole. Z Gastroenterol. 1990;28:271-274. [PubMed] |
| 26. | Klinkenberg-Knol EC, Festen HP, Jansen JB, Lamers CB, Nelis F, Snel P, Lückers A, Dekkers CP, Havu N, Meuwissen SG. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994;121:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 253] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Moayyedi P, Wason C, Peacock R, Walan A, Bardhan K, Axon AT, Dixon MF. Changing patterns of Helicobacter pylori gastritis in long-standing acid suppression. Helicobacter. 2000;5:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Stolte M, Meining A, Schmitz JM, Alexandridis T, Seifert E. Changes in Helicobacter pylori-induced gastritis in the antrum and corpus during 12 months of treatment with omeprazole and lansoprazole in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1998;12:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Kuipers EJ, Uyterlinde AM, Peña AS, Hazenberg HJ, Bloemena E, Lindeman J, Klinkenberg-Knol EC, Meuwissen SG. Increase of Helicobacter pylori-associated corpus gastritis during acid suppressive therapy: implications for long-term safety. Am J Gastroenterol. 1995;90:1401-1406. [PubMed] |
| 30. | Schenk BE, Kuipers EJ, Nelis GF, Bloemena E, Thijs JC, Snel P, Luckers AE, Klinkenberg-Knol EC, Festen HP, Viergever PP. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut. 2000;46:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Berstad AE, Hatlebakk JG, Maartmann-Moe H, Berstad A, Brandtzaeg P. Helicobacter pylori gastritis and epithelial cell proliferation in patients with reflux oesophagitis after treatment with lansoprazole. Gut. 1997;41:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 32. | Eissele R, Brunner G, Simon B, Solcia E, Arnold R. Gastric mucosa during treatment with lansoprazole: Helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology. 1997;112:707-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Furuta T, Baba S, Takashima M, Futami H, Arai H, Kajimura M, Hanai H, Kaneko E. Effect of Helicobacter pylori infection on gastric juice pH. Scand J Gastroenterol. 1998;33:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Geboes K, Dekker W, Mulder CJ, Nusteling K. Long-term lansoprazole treatment for gastro-oesophageal reflux disease: clinical efficacy and influence on gastric mucosa. Aliment Pharmacol Ther. 2001;15:1819-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Bloemena EC, Sandell M, Nelis GF, Snel P, Festen HP, Meuwissen SG. Atrophic gastritis during long-term omeprazole therapy affects serum vitamin B12 levels. Aliment Pharmacol Ther. 1999;13:1343-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Mashiba H, Sasaki N, Taniyama K. Changes in Helicobacter pylori-induced gastritis in the antrum and corpus during long-term acid-suppressive treatment in Japan. Aliment Pharmacol Ther. 2000;14:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | van Grieken NC, Meijer GA, Weiss MM, Bloemena E, Lindeman J, Baak JP, Meuwissen SG, Kuipers EJ. Quantitative assessment of gastric corpus atrophy in subjects using omeprazole: a randomized follow-up study. Am J Gastroenterol. 2001;96:2882-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Graham DY, Opekun AR, Yamaoka Y, Osato MS, el-Zimaity HM. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther. 2003;17:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Recavarren-Arce S, León-Barúa R, Cok J, Berendson R, Gilman RH, Ramírez-Ramos A, Rodríguez C, Spira WM. Helicobacter pylori and progressive gastric pathology that predisposes to gastric cancer. Scand J Gastroenterol Suppl. 1991;181:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Sipponen P. Gastric cancer--a long-term consequence of Helicobacter pylori infection. Scand J Gastroenterol Suppl. 1994;201:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Sipponen P, Kekki M, Siurala M. Atrophic chronic gastritis and intestinal metaplasia in gastric carcinoma. Comparison with a representative population sample. Cancer. 1983;52:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Sipponen P. Atrophic gastritis as a premalignant condition. Ann Med. 1989;21:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | el-Zimaity HM, al-Assi MT, Genta RM, Graham DY. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol. 1995;90:1962-1964. [PubMed] |
| 45. | Graham DY, Lew GM, Lechago J. Antral G-cell and D-cell numbers in Helicobacter pylori infection: effect of H. pylori eradication. Gastroenterology. 1993;104:1655-1660. [PubMed] |
| 46. | Kim HY, Kim YB, Park CK, Yoo JY, Graham DY. Co-existing gastric cancer and duodenal ulcer disease: role of Helicobacter pylori infection. Helicobacter. 1997;2:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Kirk RM, Jeffery PJ. Development of surgery for peptic ulcer: a review. J R Soc Med. 1981;74:828-830. [PubMed] |
| 48. | Correa P, Cuello C, Fajardo LF, Haenszel W, Bolaños O, de Ramírez B. Diet and gastric cancer: nutrition survey in a high-risk area. J Natl Cancer Inst. 1983;70:673-678. [PubMed] |
| 49. | Correa P. Diet modification and gastric cancer prevention. J Natl Cancer Inst Monogr. 1992;75-78. [PubMed] |
| 50. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] |
| 51. | Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst. 1970;44:297-306. [PubMed] |
| 52. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 53. | Kimura K, Takemoto T: An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (5)] |
| 54. | Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32 Suppl 2:329-347. [PubMed] |
| 55. | Ricuarte O, Gutierrez O, Cardona H, Kim JG, Graham DY, El-Zimaity HM. Atrophic gastritis in young children and adolescents. J Clin Pathol. 2005;58:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Takemoto T. Endoscopic diagnosis of chronic gastritis. Diagnosis and Treatment. 1966;54:1274-1285. |
| 57. | HEBBEL R. The topography of chronic gastritis in cancer-bearing stomachs. J Natl Cancer Inst. 1949;10:505-522; Disc, 526-532. [PubMed] |
| 58. | Filipe MI. Natural history of precursor lesions to gastric carcinoma: growth factors and oncogenes in the metaplasia-dysplasia-carcinoma sequence. Eur J Cancer Prev. 1994;3 Suppl 2:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Huang CB, Xu J, Huang JF, Meng XY. Sulphomucin colonic type intestinal metaplasia and carcinoma in the stomach. A histochemical study of 115 cases obtained by biopsy. Cancer. 1986;57:1370-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Filipe MI, Potet F, Bogomoletz WV, Dawson PA, Fabiani B, Chauveinc P, Fenzy A, Gazzard B, Goldfain D, Zeegen R. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985;26:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | El-Zimaity HM, Ramchatesingh J, Saeed MA, Graham DY. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol. 2001;54:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Silva S, Filipe MI, Pinho A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric ulcer. A follow up study. Gut. 1990;31:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Siurala M, Lehtola J, Ihamäki T. Atrophic gastritis and its sequelae. Results of 19-23 years' follow-up examinations. Scand J Gastroenterol. 1974;9:441-446. [PubMed] |
| 65. | Rösch W, Demling L, Elster K. Is chronic gastritis a reversible process Follow-up study of gastritis by step-wise biopsy. Acta Hepatogastroenterol (Stuttg). 1975;22:252-255. [PubMed] |
| 66. | Maaroos HI, Salupere V, Uibo R, Kekki M, Sipponen P. Seven-year follow-up study of chronic gastritis in gastric ulcer patients. Scand J Gastroenterol. 1985;20:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Ihamäki T, Kekki M, Sipponen P, Siurala M. The sequelae and course of chronic gastritis during a 30- to 34-year bioptic follow-up study. Scand J Gastroenterol. 1985;20:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Villako K, Kekki M, Maaroos HI, Sipponen P, Uibo R, Tammur R, Tamm A. Chronic gastritis: progression of inflammation and atrophy in a six-year endoscopic follow-up of a random sample of 142 Estonian urban subjects. Scand J Gastroenterol Suppl. 1991;186:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Niemelä S, Karttunen T, Kerola T. Helicobacter pylori-associated gastritis. Evolution of histologic changes over 10 years. Scand J Gastroenterol. 1995;30:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Valle J, Kekki M, Sipponen P, Ihamäki T, Siurala M. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol. 1996;31:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Filipe MI, Muñoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 295] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Kato Y, Kitagawa T, Yanagisawa A, Kubo K, Utsude T, Hiratsuka H, Tamaki M, Sugano H. Site-dependent development of complete and incomplete intestinal metaplasia types in the human stomach. Jpn J Cancer Res. 1992;83:178-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Kato Y, Sugano H. [Morphological studies on precancerous lesions of the stomach and large intestine]. Gan To Kagaku Ryoho. 1983;10:443-458. [PubMed] |
| 75. | Greibe J, Bugge P, Gjorup T, Lauritzen T, Bonnevie O, Wulff HR. Long-term prognosis of duodenal ulcer: follow-up study and survey of doctors' estimates. Br Med J. 1977;2:1572-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Ramesar KC, Sanders DS, Hopwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol. 1987;40:1287-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Oohara T, Tohma H, Aono G, Ukawa S, Kondo Y. Intestinal metaplasia of the regenerative epithelia in 549 gastric ulcers. Hum Pathol. 1983;14:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Cornaggia M, Capella C, Riva C, Finzi G, Solcia E. Electron immunocytochemical localization of pepsinogen I (PgI) in chief cells, mucous-neck cells and transitional mucous-neck/chief cells of the human fundic mucosa. Histochemistry. 1986;85:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | OI M, OSHIDA K, SUGIMURA S. The location of gastric ulcer. Gastroenterology. 1959;36:45-56. [PubMed] |
| 80. | Helpap B, Hattori T, Gedigk P. Repair of gastric ulcer. A cell kinetic study. Virchows Arch A Pathol Anat Histol. 1981;392:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Hattori T, Helpap B, Gedigk P. The morphology and cell kinetics of pseudopyloric glands. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Miwa K, Kamata T, Miyazaki I, Hattori T. Kinetic changes and experimental carcinogenesis after Billroth I and II gastrectomy. Br J Surg. 1993;80:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 83. | Savage A, Jones S. Histological appearances of the gastric mucosa 15--27 years after partial gastrectomy. J Clin Pathol. 1979;32:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Begue RE, Gonzales JL, Correa-Gracian H, Tang SC. Helicobacter pylori infection in children with abdominal ailments in a developing country. Am J Med Sci. 1997;314:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Stolte M, Vieth M. Gastritis and gastric cancer: which morphological type of Helicobacter gastritis is a precancerous risk. Chin J Dig Dis. 2005;6:110-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol. 2003;21:2070-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3624] [Article Influence: 120.8] [Reference Citation Analysis (6)] |
| 88. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 662] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 89. | Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, Murch SH. Focal-enhanced gastritis in regressive autism with features distinct from Crohn's and Helicobacter pylori gastritis. Am J Gastroenterol. 2004;99:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol. 1999;30:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Camorlinga-Ponce M, Aviles-Jimenez F, Cabrera L, Hernández-Pando R, Muñoz O, Soza J, Torres J. Intensity of inflammation, density of colonization and interleukin-8 response in the gastric mucosa of children infected with Helicobacter pylori. Helicobacter. 2003;8:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | el-Zimaity HM, Graham DY, al-Assi MT, Malaty H, Karttunen TJ, Graham DP, Huberman RM, Genta RM. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Lutze M E- Editor Liu WF