Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5628
Revised: November 10, 2005
Accepted: November 18, 2005
Published online: September 21, 2006
AIM: To investigate the intracellular apoptotic signals engaged by resveratrol in three gastric adenocarcinoma cancer cell lines, two of which (AGS and SNU-1) express p53 and one (KATO-III) with deleted p53.
METHODS: Nuclear fragmentation was used to quanti-tate apoptotic cells; caspase activity was determined by photometric detection of cleaved substrates; formation of oxidized cytochrome C was used to measure cytochrome C activity, and Western blot analysis was used to determine protein expression.
RESULTS: Gastric cancer cells, irrespective of their p53 status, responded to resveratrol with fragmentation of DNA and cleavage of nuclear lamins A and B and PARP. Resveratrol, however, has no effect on mitochondria-associated apoptotic proteins Bcl-2, Bcl-xl, Bax, Bid or Smac/Diablo, and did not promote sub-cellular redistribution of cytochrome C, indicating that resveratrol-induced apoptosis of gastric carcinoma cells does not require breakdown of mitochondrial membrane integrity. Resveratrol up-regulated p53 protein in SNU-1 and AGS cells but there was a difference in response of intracellular apoptotic signals between these cell lines. SNU-1 cells responded to resveratrol treatment with down-regulation of survivin, whereas in AGS and KATO-III cells resveratrol stimulated caspase 3 and cytochrome C oxidase activities.
CONCLUSION: These findings indicate that even within a specific cancer the intracellular apoptotic signals engaged by resveratrol are cell type dependent and suggest that such differences may be related to differentiation or lack of differentiation of these cells.
- Citation: Riles WL, Erickson J, Nayyar S, Atten MJ, Attar BM, Holian O. Resveratrol engages selective apoptotic signals in gastric adenocarcinoma cells. World J Gastroenterol 2006; 12(35): 5628-5634
- URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5628
Gastric cancer is a major cause of mortality both in developed and underdeveloped countries because currently available chemotherapeutic regimens are not very effective, resulting in high recurrence rates and poor survival. There is strong evidence that the predominant etiological factors contributing to development of gastric cancer are infections with H pylori during early years of life and/or exposure to chemical carcinogens such as those in cigarettes and cured meat[1]. Identification and eradication of H pylori in the world population would be an economically prohibitive undertaking because more than 50% of population over the age of 50 are infected with the bacterium, and eradiation would not benefit those with pre-malignant gastric mucosal alterations. However, given the epigenetic origin and prolonged onset of gastric cancer development, the concept of cancer chemoprevention presents an attractive hypothesis to reduce the risk of gastric cancer.
Since apoptosis-inducing compounds control cancer cell proliferation, it is feasible that cancer development may be arrested through molecular intervention with compounds that retard cellular proliferation and induce apoptosis. Trans-resveratrol, a polyphenol found in grapes, wine and peanuts, presents itself as a dietary chemopreventive because numerous studies have demonstrated its ability to suppress proliferation and induce apoptosis in a variety of transformed cells[2]. We have shown that gastric adenocarcinoma cells respond to resveratrol with inhibition of DNA synthesis, cell cycle arrest, suppressed proliferation, and induction of apoptosis[3,4], and there is evidence that resveratrol inhibits the growth of transplanted gastric tumor[5].
There are two major pathways for induction of apoptosis: (1) the extrinsic pathway activated when extracellular ligands interact with receptors of the TNF family (TNF, FAS, and TRAIL) and (2) the intrinsic pathway, induced by destabilization of mitochondria. Resveratrol up-regulates Fas and Fas-L in gastric adenocarcinoma cells that express p53, whereas only Fas-L becomes up-regulated in cells whose p53 is deleted[6], suggesting that apoptotic signals engaged by resveratrol within individual gastric carcinoma cell lines may be dependent on p53 status of the cell. Here we explore the action of resveratrol on intracellular apoptotic signals in three different gastric adenocarcinoma cell lines.
Resveratrol is a kind gift from Pharmascience, Montreal, Quebec, Canada. The following caspase substrates were purchased from Calbiochem, EMD Biosciences, Inc., San Diego, CA: caspase 1 substrate (Ac-WEHD-pNA), caspase 3 substrate (Ac-DEVD-pNA), caspase 6 substrate (Ac-VEID-pNA), caspase 8 substrate (Ac-IETD-pNA), and caspase 9 substrate (Ac-LEHD-pNA). Cytochrome C Oxidase Assay Kit (CYTOC-OX1) was purchased from Sigma-Aldrich, St. Louis, MO. Antibodies to cytochrome C and lamin B were obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, CA; antibodies to PARP, Bcl-xl, Bax, Bid, and cleaved Lamin A were from Cell Signaling Biotechnology, Beverly, MA; antibody to survivin was from Novus Biologicals, Inc., Littleton, CO; p53 antibody was from PharMingen, Inc/BD Biosciences, San Diego, CA; antibody to β-actin was from Sigma-Aldrich, St. Louis, MO, and antibodies to Bcl-2 and Smac/DIABLO were from Calbiochem/EMD Biosciences, Inc., La Jolla, CA. Chemiluminescence detection system (ECL Western Blotting System) was from Amersham/Pharmacia Biotech, Piscataway, NJ.
Human gastric adenocarcinoma cell lines AGS (ATCC: CLR-1739), SNU-1 (ATCC: CRL-5971) and KATO-III (ATCC: HTB 103) were routinely cultured in RPMI-1640 media supplemented with 100 mL/L fetal bovine serum (FBS), 10 U/mL of streptomycin and 0.25 mg/L of amphotericin B at 37°C in humidified air with 50 mL/L CO2. Cells were allowed to equilibrate in fresh media for 2-3 h prior to addition of resveratrol which was dissolved in 950 mL/L ethanol. The concentration of ethanol was always maintained at 0.1% in both treated and untreated cells. A stock solution of 100 mmol/L resveratrol was prepared weekly and stored in the dark at -20°C.
Percent of apoptotic cells was determined using Cell Death Detection ELISAPLUS kit from Roche Applied Science, Indianapolis, IN. Cells (1 × 104 cells/200 μL) were incubated for designated times with or without 100 μmol/L resveratrol, and percent of apoptotic cells was calculated based on 100% apoptosis obtained after 48 h of cell exposure to 50 μmol/L camptothecin.
Cells were plated in fresh media at 1 × 106 cells/5 mL in 6 well plates, allowed to equilibrate for 3 h, and treated with 100 μmol/L resveratrol for 24 or 48 h. Vehicle was added to untreated controls that were also cultured for 24 or 48 h. At the end of the specified incubation time, cells were harvested, washed once with 5 mL of PBS and lysed for 30 min at 4°C in lysis buffer A (10 mmol/L Tris pH 7.4, 150 mmol/LNaCl, 1 mmol/L EDTA, 1 mmol/LEGTA, 1% Triton X-100, 0.2 mmol/L sodium vanadate, 2 mmol/L phenylmethyl-sulfonyl fluoride, 0.5% NP-40, and 20 mmol/L NaF). The caspase assay was performed in 96 well plates in a total volume of 100 μL as follows: to 32 μL of assay buffer (312.5 mmol/L HEPES, 31.25% glucose, 0.3125% CHAPS) were added 2 μL of DMSO, 10 μL of 100 mmol/L DTT, 30 μg of cell lysate protein, and the volume adjusted with deionized water to 98 μL. Following addition of 2 μL of 10 mmol/L fluorogenic peptide substrate, specific for each caspase, the reaction was incubated at 25°C for 4 h after which time absorbance measured at 405 nm on a microplate reader (Molecular Devices Corporation, Sunnyvale, CA). Activity of each caspase activity was calculated from a standard curve of p-nitroaniline (pNA) absorbance at 405 nm and values are expressed as pmoles of pNA generated per mg lysate protein. Protein content of cell lysates was determined by the method of Lowry[7].
Cellular levels of cytochrome C, p53, survivin, Bax, Bcl-2, Bcl-xl, Bid, Smac/DIABLO, cleaved lamin A, lamin B, PARP, and β-actin (used as control for equal protein loading) were determined in untreated and resveratrol treated cell fractions by Western blot detection. To test the action of resveratrol, cells (0.2 × 109 cells/L of media) were treated with 100 μmol/L resveratrol for 24 or 48 h, after which time they were harvested, washed with PBS, suspended in 0.5 mL of lysis buffer B (10 mmol/L Tris pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 0.2 mmol/L sodium vanadate, 2 mmol/L phenylmethyl-sulfonyl fluoride, 0.5% NP-40, 20 mmol/L NaF, and 2 mg/L leupeptin), and rapidly frozen in liquid nitrogen. Cells were lysed by a freeze-thaw cycle in liquid nitrogen followed by 30 min at 4°C and cell cytosol was prepared from cell lysates by centrifugation at 10 000 ×g for 20 min. Mitochondria were prepared from whole cells by a subcellular fractionation protocol[8] and protein content of each fraction determined by the Lowry method[7]. Equal amounts of protein were separated by SDS-PAGE on either 15% resolving Tris-HCl gels for detection of cytochrome C, survivin, Bcl-2, Bcl-xl, Bax, Bid, and Smac/DIABLO or 12% gels for detection of p53, PARP, cleaved lamin A, lamin B, β-actin, followed by transfer to PVDF membranes, and antigen detected by chemiluminescence. Depicted results are representative of at least two individual experiments.
Cytochrome C oxidase activity was measured in cell lysates from untreated and resveratrol treated cells using a commercially available kit from Sigma-Aldrich. Activity of cytochrome C oxidase was determined by measuring the conversion of reduced cytochrome C, which absorbs light at A50, to its oxidized form that does not absorb light at this wavelength, and values are expressed as ΔA550 generated by 35 μg of lysate protein.
Numerical data were analyzed for statistical significance by Student’s t test, and Results are expressed as the mean ± SE. Statistical significance is denoted as P < 0.01 and P < 0.05.
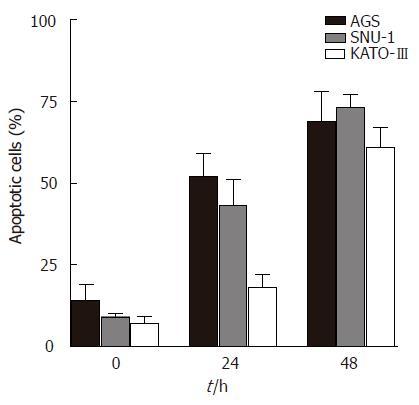
Gastric adenocarcinoma SNU-1 cells (expressing p53) and Kato-III cells (p53 deleted) respond to resveratrol treatment with decreased proliferation, concentration dependent inhibition of DNA synthesis and cell cycle arrest[3,4]. To determine whether p53 status of the cell regulates the action of resveratrol on apoptosis, we measured the extent of apoptosis in three gastric adenocarcinoma cells lines: AGS (expressing wild type p53), SNU-1 (expressing p53) and KATO-III. Results show that cell treatment with 100 μmol/L resveratrol induces a time dependent apoptosis in all three cell lines (Figure 1). Although a small percentage of apoptotic cells was present in the absence of resveratrol, treatment with 100 μmol/L resveratrol for 24 h resulted in significantly increased accumulation of apoptotic cells, and treatment for 48 h further increasing the percentage of apoptotic cells. After 48 h of treatment with 100 μmol/L resveratrol nearly 70% of AGS cells, 75% of SNU-1 cells and 62% of Kato-III cells became apoptotic.
Apoptosis is characterized by loss of nuclear integrity and degradation of DNA and we previously demonstrated that exposure of gastric adenocarcinoma cells to resveratrol results in DNA fragmentation[3,4]. Here we determined the action of resveratrol on nuclear proteins PARP, lamin A and lamin B (Figure 2A), and show that resveratrol promotes their cleavage in all three cell lines. Increased levels of lamin A cleavage products were seen in all cells within 24 h of resveratrol treatment, whereas lamin B cleavage products were seen in AGS cells after 24 h, while SNU-1 and Kato-III cells required 48 h of exposure to 100 μmol/L resveratrol to induce lamin B cleavage. Resveratrol treatment caused cleavage of the 116 kDa PARP to an 89 kDa fragment in all three cell lines although there was a time difference among the individual cell lines. In SNU-1 cells the 89 kDa breakdown product of PARP was detected after 24 h while in AGS and KATO-III cells PARP breakdown product was seen after 48 h. Closer analysis of PARP in SNU-1 cells revealed presence of the 89 kDa fragment within 6 h after cell exposure to resveratrol with further breakdown of the 89 kDa band to a smaller fragment after prolonged treatment and nearly total loss of the 116 kDa PARP protein after 72 h of cell exposure to 100 μmol/L (Figure 2B).
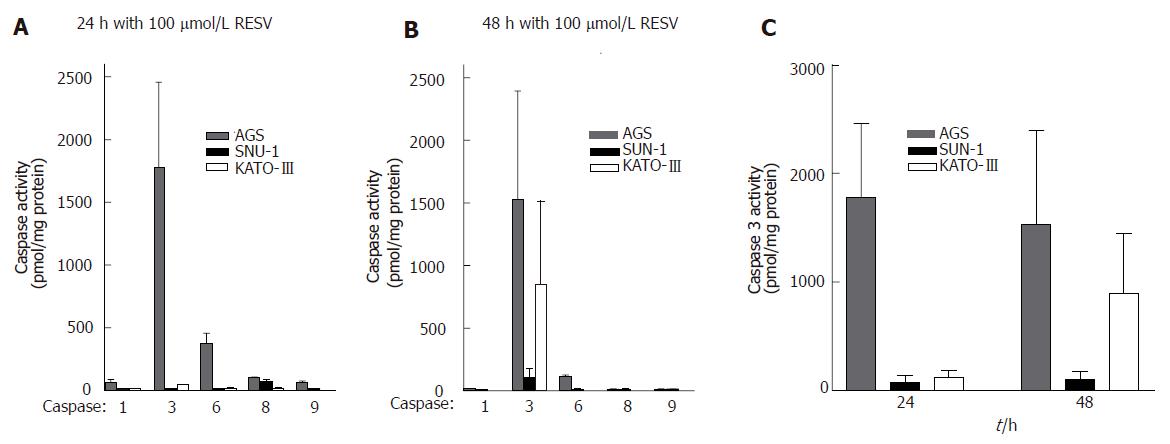
Because nuclear proteins become cleaved during resveratrol-induced apoptosis, we investigated whether this action of resveratrol results from activation of caspases. Activities of caspases 1, 3, 6, 8, and 9 were measured in untreated cells and in cells treated for 24 or 48 h with 100 μmol/L resveratrol. Results show that 100 μmol/L resveratrol had no effect on caspases 1, 8, and 9, but significantly stimulated caspase 3 activity in AGS and KATO-III cells and caused some activation of caspase 6 in AGS cells after 24 h. Caspase 3 activity in AGS cells was increased after 24 h of treatment and remained elevated after 48 h, whereas in KATO-III cells caspase 3 activity responded to resveratrol only after 48 h. Resveratrol, however, had no effect on caspase 3 activity in SNU-1 cells (Figure 3).
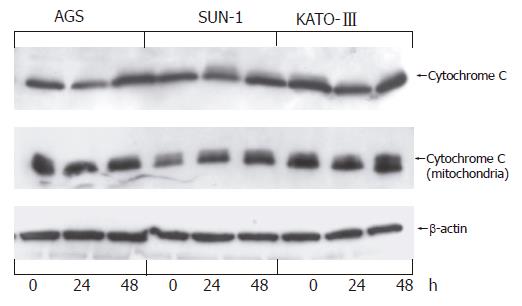
Numerous cytotoxic reagents, radiation, and growth factor withdrawal induce apoptosis by promoting release of mitochondrial cytochrome C into cell cytosol. To determine whether resveratrol targets mitochondrial permeability in gastric adenocarcinoma cells we measured distribution of cytochrome C protein between cell cytosol and mitochondria after 24 and 48 h of cell treatment with 100 μmol/L resveratrol. Results indicate that cytochrome C was found to be present in both mitochondria and cytosol of each cell line prior to treatment with resveratrol, and resveratrol treatment had no further effect on redistribution of cytochrome C protein between these two compartments (Figure 4).
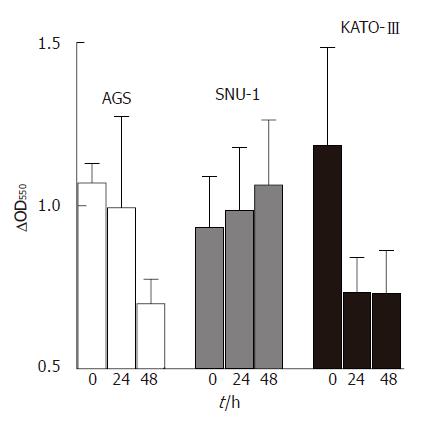
In addition to its role in apoptosis, cytochrome C functions in the respiratory chain as carrier of electrons from flavoproteins to cytochrome oxidase. Since resveratrol is a known antioxidant and inhibits mitochondrial respiratory chain[9], we inquired whether cytochrome C contributes to the antioxidant potential of resveratrol by measuring cytochrome C oxidase activity in untreated as well as resveratrol treated cells. Results show that resveratrol had a significant stimulatory effect on cytochrome C oxidase activity in KATO-III cells after 24 h of cell treatment, and in AGS cells after 48 of treatment, but had no effect on cytochrome C oxidase activity in SNU-1 cells (Figure 5).
Cellular levels of survivin, an inhibitor of apoptosis, have been shown to correlate inversely with expression of the p53 tumor suppressor[10-12], and we determined the action of resveratrol on survivin and p53 protein levels in AGS, SNU-1 and Kato-III cells. Results presented in Figure 6 indicate that resveratrol up-regulates p53 protein in AGS and SNU-1 cells but has no effect on the p53 status in Kato-III cells. Within 24 h after exposure to 100 μmol/L resveratrol survivin levels in SNU-1 cells became down-regulated. Although AGS cells express p53 and its p53 is up-regulated by resveratrol, levels of survivin in AGS cells were not altered by resveratrol, and resveratrol had no effect on survivin in KATO-III cells.
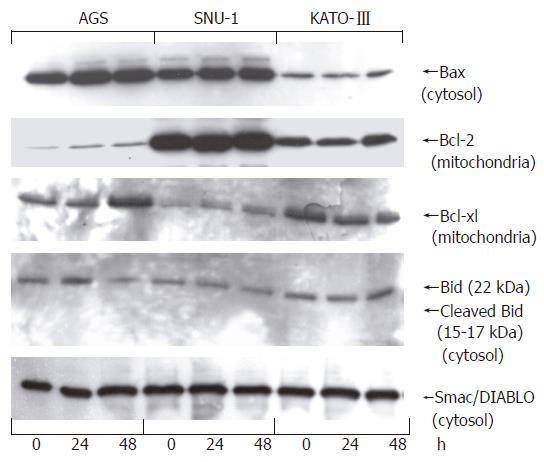
To evaluate the contribution of pro- and anti-apoptotic Bcl-2 proteins in the response of gastric carcinoma cells to resveratrol we measured protein levels of anti-apoptotic Bcl-2 and Bcl-xl and pro-apoptotic Bax, as well as Bid and Smac/DIABLO. Cells were treated with 100 μmol/L resveratrol for 24 or 48 h, sub-cellular fractions prepared as described in Methods, and protein levels determined by Western blotting. Isolated mitochondria were used to determine Bcl-2 and Bcl-xl levels, whereas Bax, Bid and Smac/DIABLO were measured in cell cytosol. Results presented in Figure 7 show that cell treatment with 100 μmol/L resveratrol for up to 48 h had no effect on Bax, Bcl-2, Bcl-xl, and Smac/DIABLO, and did not promote cleavage of cytosolic 22 kDa Bid to the active 15-17 kDa form.
A common feature of malignant cells is their ability to proliferate without restraint and, therefore, apoptosis is considered the predominant pathway for elimination of malignant cells with the signals engaged by an apoptotic agent determining its efficacy as a chemopreventive or as an adjuvant to chemotherapy. Previous work from this laboratory has shown that resveratrol-induced engagement of Fas receptor is dependent on the p53 status of the cells. The aim of the present investigation was to determine whether intracellular apoptotic signals engaged by resveratrol are also regulated to some extent by p53. To enhance the signaling response to resveratrol all experiments were performed using 100 μmol/L because this concentration has been repeatedly shown to promote significant apoptotic response in human gastric adenocarcinoma cells[3-5] and in other malignant cells[13-15].
One of the differences among the three gastric carcinoma cell lines used in this study is their p53 status: Kato-III cells do not express p53, whereas p53 is present in both AGS and SNU-1 cells. Resveratrol induced a time-dependent apoptotic response in all three cell lines irrespective of their p53 status, and in each cell line resveratrol-induced apoptosis was associated with cleavage of PARP, lamin A and lamin B. Other studies investigating the involvement of p53 in cellular response to resveratrol have also shown that resveratrol induces apoptosis and up-regulates p53 in cells that express this tumor suppressor[16,17], and that it induces apoptosis in p53 deficient cells[18,19]. Tumor suppressor p53 exerts control over proliferation and apoptosis by initiating transcriptional activation of specific genes, among them the gene for survivin[10-12], an inhibitor of apoptosis found to be expressed in all types of malignancies but not in normal, differentiated cells. Patients with gastric cancer express increased abundance of survivin[20] and survivin expression correlates with p53 accumulation[18], whereas suppression of survivin inhibits growth of gastric cancer cells and decreases tumorigenesis[21]. In our study resveratol increased p53 levels in both AGS and SNU-1 cells, but only p53-expressing SNU-1 cells responded to resveratrol treatment with loss of survivin suggesting that p53 in AGS cells may have mutations, a common finding in tumor cells.
Since resveratrol is a small, lipophylic molecule it can intercalate within the mitochondrial membrane and directly induce apoptosis through destabilization of mitochondrial membrane. To address this question we determined action of resveratrol action on Bcl-2 family of apoptotic regulators. These proteins control mitochondrial permeability and promote release of mitochondrial cytochrome C. Our findings revealed that resveratrol had no effect on either anti-apoptotic Bax or pro-apoptotic Bcl-2 and Bcl-xl, did not promote cleavage of Bid and had no effect on subcellular redistribution of cytochrome C. On the basis of these results we conclude that resveratrol-induced apoptosis of gastric cancer cells in culture is not an outcome of mitochondrial integrity breakdown. The action of resveratrol on Bcl-2 proteins has been investigated in a number of cells with results supporting a cell type-dependent response. Human leukemia U937 cells readily succumbed to apoptosis after treatment with 100 μmol/L resveratrol, whereas apoptosis was significantly inhibited when these cells were modified to over-express Bcl-2[22]. In non-Hodgkin’s lymphoma and multiple myeloma cell lines Bcl-xl expression was down-regulated by resveratrol[23], but resveratrol had no effect on Bcl-xl, Bcl-2 nor Bax in other malignant cells[24], and Bax levels remained unchanged in colorectal carcinoma cells after 24 h of treatment with 40 μmol/L resveratrol[25]. Since resveratrol caused a decrease in the ratio of Bcl-2/Bax in transplanted gastric tumors and we can only conclude that cells in culture respond differently to resveratrol from primary gastric cancer cells transplanted into nude mice.
Destabilization of mitochondria usually results in release of mitochondrial cytochrome C into cells cytosol, but we found no evidence of involvement of mitochondria destabilizing Bcl-2 proteins in resveratrol-induced apoptosis of gastric carcinoma cells. However, we did observe presence of cytochrome C in cytosol of untreated as well as resveratrol treated cells. Cytosolic cytochrome C has also been identified within secretory granules of normal rat pancreas and anterior pituitary[26], and because secretory granules are present in gastric carcinoma cells[27,28], we addressed the possible role of cytosolic cytochrome C by determining cytochrome C oxidase activity. Our data revealed that resveratrol stimulates cytochrome C oxidase activity in AGS and KATO-III cells, an action that would contribute to the antioxidant potential of these cells. Since transformed cells generate low levels of reactive oxygen that activate gene transcription and stimulate their proliferation[29,30], antioxidants suppress generation of endogenous reactive oxygen and are considered antiproliferative. We previously demonstrated that resveratrol is antiproliferative and behaves as an antioxidant in gastric adenocarcinoma cells[3,4]. Our current finding that resveratrol stimulates cytochrome C oxidase is a feasible mechanism for its antioxidant and antiproliferative action toward these. Inhibition of proliferation and induction of apoptosis are known to be closely related events, and when activation of cytochrome C oxidase is coupled with increased casapse 3 activity, as was seen in AGS and KATO-III cells, these events might provide sufficient stimuli to inhibit cellular proliferation and induce apoptosis. Although resveratrol had no effect on either cytochrome C oxidase or caspase 3 in SNU-1 cells, apoptosis in SNU-1 cells probably results from down-regulation of survivin and removal of its protective mechanisms, as was demonstrated for human bladder cells[31].
In summary, our results reveal that individual gastric carcinoma cell lines respond to resveratrol with engagement of individual apoptotic signals. In p53 expressing SNU-1 cells resveratrol up-regulated p53 and down-regulated survivin, whereas in KATO-III cells and in AGS cells resveratrol stimulated caspase 3 and cytochrome C oxidase activities, enabling suppression of proliferation while stimulating breakdown of nuclear proteins. These findings indicate that even within a specific disease resveratrol can engage alternate apoptotic targets thus providing further evidence that resveratrol can be considered a versatile chemopreventive agent.
S- Editor Wang J L- Editor Ma JY E- Editor Bai SH
| 1. | Blankfield RP. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2002;346:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Atten MJ, Attar BM, Milson T, Holian O. Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C-mediated mechanism. Biochem Pharmacol. 2001;62:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Holian O, Wahid S, Atten MJ, Attar BM. Inhibition of gastric cancer cell proliferation by resveratrol: role of nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002;282:G809-G816. [PubMed] |
| 5. | Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280-284. [PubMed] |
| 6. | Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs. 2005;23:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 8. | Rice EJ, Lindsay G. Subcellular fractionation of mitochondria. Subcellular Fractionation: A practical approach. Oxford: Oxford Univ Press 1997; 107-142. |
| 9. | Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87-97. [PubMed] |
| 10. | Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808-1812. [PubMed] |
| 11. | Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247-3257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 586] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Grossman D, Kim PJ, Blanc-Brude OP, Brash DE, Tognin S, Marchisio PC, Altieri DC. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J Clin Invest. 2001;108:991-999. [PubMed] |
| 13. | Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3633] [Cited by in RCA: 3423] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 14. | Godichaud S, Krisa S, Couronné B, Dubuisson L, Mérillon JM, Desmoulière A, Rosenbaum J. Deactivation of cultured human liver myofibroblasts by trans-resveratrol, a grapevine-derived polyphenol. Hepatology. 2000;31:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Huang C, Ma WY, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Laux MT, Aregullin M, Berry JP, Flanders JA, Rodriguez E. Identification of a p53-dependent pathway in the induction of apoptosis of human breast cancer cells by the natural product, resveratrol. J Altern Complement Med. 2004;10:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer. 2001;94:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Bernhard D, Tinhofer I, Tonko M, Hübl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ. 2000;7:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Yu J, Leung WK, Ebert MP, Ng EK, Go MY, Wang HB, Chung SC, Malfertheiner P, Sung JJ. Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer. 2002;87:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, Zou B, Zhu YB, Jiang SH, Wong WM. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63:7724-7732. [PubMed] |
| 22. | Park JW, Choi YJ, Suh SI, Baek WK, Suh MH, Jin IN, Min DS, Woo JH, Chang JS, Passaniti A. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis. 2001;22:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71-84. [PubMed] |
| 24. | Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Mahyar-Roemer M, Köhler H, Roemer K. Role of Bax in resveratrol-induced apoptosis of colorectal carcinoma cells. BMC Cancer. 2002;2:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Soltys BJ, Andrews DW, Jemmerson R, Gupta RS. Cytochrome-C localizes in secretory granules in pancreas and anterior pituitary. Cell Biol Int. 2001;25:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Koyama S, Ebihara T, Osuga T. Histologic and immunohistochemical studies of alpha-fetoprotein (AFP)-producing gastric carcinoma. Gastroenterol Jpn. 1987;22:419-427. [PubMed] |
| 29. | Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1216] [Article Influence: 41.9] [Reference Citation Analysis (7)] |
| 30. | Irani K, Goldschmidt-Clermont PJ. Ras, superoxide and signal transduction. Biochem Pharmacol. 1998;55:1339-1346. [PubMed] |
| 31. | Tyagi AK, Agarwal C, Singh RP, Shroyer KR, Glode LM, Agarwal R. Silibinin down-regulates survivin protein and mRNA expression and causes caspases activation and apoptosis in human bladder transitional-cell papilloma RT4 cells. Biochem Biophys Res Commun. 2003;312:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |