INTRODUCTION
H pylori is one of the most common human pathogens, since it infects the gastric mucosa of about 50% of the world’s population. The majority of infections are asymptomatic, making the infection lifelong without effective bacterial eradication. The clinical magnitude of this bacterium has become accepted rather recently. H pylori has been recognized as the causal agent for chronic gastritis and gastric and duodenal ulcers. Additionally, epidemiological and statistical studies associated the infection with a higher risk of gastric malignancy leading the World Health Organization International Agency for Research in Cancer to categorize H pylori as a class I carcinogen.
The bacteria induce a host immune response, but the persistence of the infection suggests that the response is not effective in eliminating the infection. Furthermore, multiple lines of evidence suggest that the immune response contributes to the pathogenesis associated with the infection. As a result, the immune response induced by H pylori is a subject of continuous study that has encouraged numerous questions. The inability of the host response to clear infections with H pylori could reflect down-regulatory mechanisms that limit the resulting immune responses to prevent harmful inflammation as a means to protect the host. Consequently, the chronic immune response induced may be inadequate or misdirected, and could thus afford a colonization advantage for the bacteria by providing improved availability of adhesion places. An example of this is the resulting increase in class II major histocompatibility complex (MHC) and CD74, induced by IFN-γ and IL-8, that are used as receptors by H pylori[1-3].
H pylori has been shown to employ multiple mechanisms to antagonize, impair, or subvert host responses[4]. For instance, H pylori has been noted to inhibit macrophage nitric oxide production and phagocytosis[5]. Another mechanism where H pylori can down-regulate the immune response is through its VacA virulence factor. This cytotoxin can interfere with the processing and presentation of antigens by antigen-presenting cells (APCs)[6], and can also inhibit T cell activation through interference of the calcineurin-associated IL-2 signaling pathway[7]. These and multiple other observations on the nature of the immune response during H pylori infection have led to models that help explain how the bacteria could persist in the gastric environment by generating a non-effective immune response. The ineffective response, together with the host factors, determines the severity of the disease.
HUMORAL RESPONSE
Nearly everyone infected with H pylori develops specific antibodies, which are found in serum and in gastric aspirates or extracts of stomach. Accordingly, elevated titers of IgG and IgA antibodies directed at membrane proteins (MP), flagelin, urease, lipopolysaccharide (LPS) and H pylori adhesin A (HpaA) have been reported in patients infected with H pylori[8,9]. Yet, those titers do not differ between asymptomatic patients and patients with duodenal ulcers. IgM- and IgA-producing cells in biopsies from the antral region of the H pylori-infected patients’ stomaches were 40- to 50-fold higher in frequency than in non-infected subjects. However, IgG-producing cell numbers are the same for non-infected and infected H pylori subjects. Those results suggest that the infection induces a large recruitment of immune cells into the gastric mucosa, particularly IgA-producing cells. A recent immunoproteome analysis compared individual sera from H pylori-positive patients suffering from gastric adenocarcinoma or duodenal ulcer with a pool of five sera from H pylori-negative patients to detect antigenic proteins from three separate H pylori strains[10]. That study recognized 30 antigens detected by H pylori positive sera, nine of these were newly identified and 21 established previously. The study established the presence of antigens related to specific disease. Interestingly, cancer sera provided stronger immunoreactivity while a similar study suggested that sera from ulcer patients have more anti- H pylori antibodies than sera from gastritis patients[11].
Due to the plasticity of the H pylori genome as well as the phase variation that the bacteria present in its LPS, specifically mimic Lewis antigens, 20% to 30% of the people infected with H pylori develop autoantibodies, with most of them specific to the gastric proton pump located in the parietal cells. These antibodies may block pump function, leading to achlorhydria associated with the infection, which contributes to the gastric damage seen during infection.
T-CELL RESPONSE
H pylori induce the recruitment of CD4+ and CD8+ T-cells into the gastric mucosa, but there appears to be preferential activation of CD4+ cells rather than CD8+ cells[12]. Several studies have noted that the T helper cell response to H pylori is polarized, since CD4+ T cells in the gastric mucosa of infected individuals produce the Th1 cytokines, interleukin (IL)-12 and interferon (IFN)-γ, whereas IL-4, a Th2 cytokine, production by these T cells is absent[13,14]. A recent study by Amedei et al[15] suggested that H pylori neutrophil-activating protein (HP-NAP) contributes to this Th1-polarized T cell response in the gastric mucosa of H pylori-infected patients. In that study, addition of HP-NAP to antigen-induced T cell lines in culture resulted in a shift from a predominant Th2 to a Th1 phenotype of specific T cells.
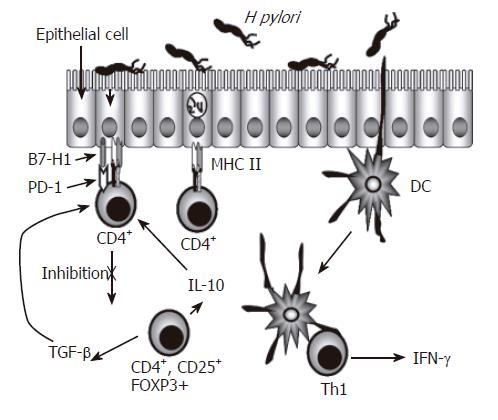
Another subset of CD4+ T cells are T regulatory cells, which produce IL-10 and transforming growth factor (TGF)-β[16]. While there is a demonstrated infiltration of T cells in the gastric mucosa and most of those are CD4+ T cells with markers of activation, various studies have tried to address the inefficiency of the host response in clearing the infection. Different studies have demonstrated that H pylori infection can decrease T cell responses as well as induce T cell anergy[12]. CD45RO+ memory T cells as well as activated CD69+ and CD25+ T cells are increased in the antral lamina propria of infected subjects[17]. Memory T cells isolated from peripheral blood from infected people responded less to stimulation with H pylori antigens than cells isolated from non-infected subjects[12,18,19]. These results suggested the presence of regulatory T (T reg) cells, CD4+ and CD25+ in the peripheral blood of H pylori infected individuals, which could inhibit the response of CD4+ T cells to H pylori. This notion was supported by observations that a higher responsiveness was obtained after depletion of H pylori-specific T reg cells[20,12]. Hence, these observations may help explain the inability of the host response to eliminate the infection due to the activation of T reg cells, which were recently reported to be increased in the gastric mucosa of H pylori-infected individuals and were described as CD4+, CD25high and FOXP3+. Such cells may simultaneously reduce mucosal damage mediated by T cells as well as reduce specific T cell responses, possibly by reducing activation of IFN-γ-producing CD4+ T cells that can be effective in protection against the infection with these bacteria. Figure 1 illustrates the presence of the various T cell populations in the infected gastric mucosa and how they may interact with one another and with other resident cells.
Figure 1 Regulation of CD4+ T Cells During H pylori Infection.
CD4+ T cell numbers increase in the gastric lamina propria of individuals infected with H pylori. These cells are predominantly Th1 cells characterized by their production of IFN-γ. Because the epithelium separates H pylori from CD4+ T cells, and also expresses key proteins associated with antigen presenting cells, the gastric epithelium, in addition to dendritic cells, could be involved in the presentation of antigens to these CD4+ T cells. The expression of inhibitory B7 related molecules along with CD4+ T cells with a regulatory T cell phenotype could be playing a role in limiting the function of effector CD4+ T cells.
The role of CD8+ T cells in the gastric mucosa of H pylori-infected individuals is less clear than that of the CD4+ T cells. Although their numbers are also increased, the CD8+ T cells that are found in the infected tissue are thought to be intraepithelial lymphocytes. Their contribution to the local response is in the form of IFN-γ production, which in turn helps increase class II MHC molecule expression on adjacent cells. A recent report by Azem and colleagues showed that H pylori-reactive CD8+ T cells can be efficiently stimulated by H pylori antigen-pulsed B cells and DCs, and that most of the CD8+ T cells in the infected gastric mucosa are memory T cells[21].
ANTIGEN PRESENTING CELLS IN THE GASTRIC MUCOSA
The activation of CD4+ T cells requires their effective cross talk with cells that express class II MHC molecules, which are classically referred to as antigen presenting cells (APC). Conventional APC include macrophages, dendritic cells and B cells. The role of these cells in the adaptive response is to internalize foreign antigens and present them in the form of peptides bound to class II MHC molecules to the T cells. The infected gastric mucosa contains a significant macrophage population that produces nitric oxide, IL-6, IL-1β, TNF-α, and IL-12 that help drive a T helper 1 response responsible for the production of IFN-γ, and little or no IL-4 and IL-5[22,23]. Although in smaller numbers, dendritic cells are also present and respond to H pylori with the production of IL-6, IL-8, IL-10 and IL-12, and have increased expression of CD80, CD83, CD86, and HLA-DR as a result of their stimulation with H pylori[24].
For efficient T cell activation, T cells require not only the T cell antigen receptor (TCR)-mediated signaling, but also costimulatory signals provided by APC[25]. The B7 family of molecules provides signals that are critical for both stimulating and inhibiting T cell activation. Engagement of CD28 by CD80 (B7-1) and CD86 (B7-2) stimulates and sustains T cell responses, whereas engagement of CTLA-4 by the same ligand inhibits T cell responses[26]. Recently, several new members of the B7 family have been identified. B7-H2 (homologue 2 also known as GL50, B7h, B7RP-1 and LICOS) has been identified as a ligand for the CD28 family member ICOS (inducible T-cell co-stimulator). Two additional B7 family members, Programmed Death-Ligand 1, PD-L1 (B7-H1) and PD-L2 (B7-DC) bind to the receptor Programmed Death-1 (PD-1) and their interaction down regulates T cell activation[27]. PD-1 is a type I transmembrane receptor expressed on activated T and B cells. Like CTLA-4, PD-1 contains an immunoreceptor tyrosine based inhibitory (ITIM) motif in its cytoplasmic region and acts as a negative regulator of lymphocyte function via multiple mechanisms, including cell-cycle inhibition and apoptosis. The literature suggests that there is another unidentified receptor for B7-H1 and B7-DC whose function has yet to be determined. Two other receptors of the B7 family are B7-H3 and B7-H4 (also known as B7S1 and B7x); however, their receptors and functions are still unclear[28-30]. In an attempt to examine whether changes in the expression of these novel B7 family members could contribute to the hyporesponsiveness of T cells in the infected gastric mucosa, we examined by real-time PCR the expression of the message for these molecules in gastric biopsies and observed the expression of B7-H1, B7-DC, and B7-H3. Since, as discussed below, the epithelium is exposed to both H pylori and T cells in the lamina propria, we examined the epithelium for these molecules and detected their expression by PCR and by Western blot analysis. B7-H1 was the most prominent coinhibitory molecule of the B7 family whose expression was induced following H pylori infection. More interesting, epithelial cells in gastric biopsies infected with H pylori showed higher B7-H1 expression compared with uninfected samples[31]. Gastric epithelial cells were found to constitutively express B7-H1, and the level of expression increased significantly during infection. T cells cocultured with gastric epithelial cells exposed to H pylori had a lower proliferation index, IL-2 secretion, and CD69 expression in response to activation via CD3. However, blockage of B7-H1 with specific anti-B7-H1 antibodies restored the responses to levels close to those of T cells not cocultured with gastric epithelial cells. This may represent a novel mechanism of immune avoidance used by H pylori, which involves the induction of coinhibitory molecule expression on gastric epithelial cells by the bacterium.
Recent elegant studies by Anderson and colleagues showed the importance of CTLA-4 in establishing T cell anergy during H pylori infection in a murine model. In this model of H pylori infection, the mice that received anti-CTLA-4 Fabs responded to an H pylori challenge with much greater inflammation and drastically decreased bacterial numbers. Their results suggested that CTLA-4 engagement may represent yet another mechanism of inactivation of H pylori-specific T cells during H pylori infection, which could in turn contribute to the chronicity of this infection[32].
While direct interaction between APC and T cells represent the traditional mechanism leading to T cell activation, another mechanism that is under active investigation involves exosomes secreted by APC. Exosomes are small membrane vesicles derived from late endosomes, which are released into the extracellular membrane and interact with membranes of other cells at a relative distance. Exosomes secreted by APC carry class I and II MHC molecules, costimulatory molecules, and adhesins. Thus, they have immunomodulatory capacity, such as in the activation of naïve T cells[33]. They have been shown to stimulate T cells in vitro and to induce anti-tumor responses in vivo[34,35]. While they are not yet characterized in the context of the T cell response to H pylori, their contribution in modulating the local response has to be considered.
Human dendritic cells have been shown to produce IL-8, IL-10, and IL-12 in response to H pylori as well as to purified H pylori antigens[36,37]. Thus, H pylori can bind to the dendritic cell receptor DC-specific ICAM-3-grabbing nonintegrin (SIGN) through the blood group Lewis X antigen present in its LPS[38]. This interaction can alter the T helper balance and favor pathogen persistence. Also, in monocytes, urease and HSP60 have been shown to be potent activators of proinflammatory cytokines via NF-κB activation[39,40].
THE GASTRIC EPITHELIUM AS AN ACTIVE PLAYER IN THE MUCOSAL RESPONSE
In terms of providing protection, the gastric epithelium has typically been regarded as a physical barrier; however, multiple studies have provided evidence to suggest that the gastric epithelium plays a key role in the inflammatory and immune responses induced by H pylori. The epithelium is the only cell phenotype in the gastric mucosa that is in direct contact with the pathogen. This feature places the epithelium in a strategic situation to interact with H pylori and with the immune elements in the lamina propria. There is strong evidence to suggest that the gastric epithelium is an active player in the response while performing functions associated with antigen presenting cells[41,42]. In addition, it is well documented that the epithelium has the ability to produce cytokines that trigger the recruitment of inflammatory cells into the gastric lamina propria[1]. The production of IL-8 in response to H pylori infection is one of the first epithelial responses. This chemokine recruits immunological components into the gastric mucosa from the periphery, particularly polymorphonuclear cells, which contribute to epithelial damage[43]. Macrophages also contribute to epithelial damage by producing nitric oxide in response to H pylori urease leading to the induction of additional inflammatory mediators[44]. However, the bacteria produce an arginase encoded by the gene rocF that competes with the NOS for L-arginine and converts this to urea and L-ornithine rather than NO[45].
One of the major mechanisms of IL-8 induction by epithelial cells is through the injection of CagA into gastric epithelial cells by a type IV secretion system[46]. This system releases CagA into the epithelial cells cytosol inducing cell proliferation and IL-8 production[47]. Our group has recently described the interaction of H pylori with CD74 on gastric epithelial cells (GEC) leading to the production of IL-8, via NF-κB activation[1]. Interestingly, IL-8 induced by H pylori, in addition to its effect in the recruitment of inflammatory cells, also acts in an autocrine manner and induces further expression of CD74[2]. This, in turn, suggests that H pylori has the ability to induce the increased expression of receptors on the host epithelium to enhance colonization and the stimulation of proinflammatory responses. As part of the inflammatory response, we noted that the H pylori-infected gastric epithelial cells produce macrophage migration inhibitory factor (MIF), which is an important cytokine that bridges the innate and adaptive immune responses[48]. The production of MIF was found to be dependent on CagA, since CagA-deficient mutant H pylori strains had a significantly reduced ability to stimulate MIF production.
Some of the interactions of the epithelium with H pylori can be detrimental to the integrity of the epithelium. For instance, we have shown that H pylori use class II MHC as receptors on GECs, and this interaction leads to apoptosis[3]. This interaction is mediated via H pylori urease. It has also been reported that cag genes may up-regulate Fas ligand (FasL) expression leading T cells to undergo apoptosis[49]. Thus, the contribution of the gastric epithelium in influencing the adaptive response by expressing molecules that either directly or indirectly limit T cell activity has to be considered in our ongoing efforts to understand the host response to H pylori.
THE INNATE RESPONSE TO H PYLORI
Other potential interactions that lead to production of pro-inflammatory cytokines include that of H pylori with toll-like receptors (TLR) expressed by epithelial cells. It has been reported that gastric epithelial cells express TLR2, TLR4, TLR5, and TLR9[50-53] that interact respectively with lipoproteins, LPS, flagellin, and CpG motifs. The expression of those receptors by epithelial cells is of importance in innate immunity against H pylori. Since these innate receptors may elicit cytokine secretion when they bind their ligands, they may have an indirect effect in the subsequent adaptive response through the enhancement of processing and presentation of antigen by host cells. However, it has been demonstrated that H pylori LPS has a 500-1000 fold lower endotoxic activity than LPS from S. typhimurium and E. coli[54,55]. This low stimulatory potential can be attributed to the phosphorylation pattern and the LPS’ lipid A acylation[55]. In addition, H pylori LPS has low binding affinity to LPS-binding protein (LBP) and in consequence, has a lower transfer rate to CD14 present in macrophages and monocytes[56].
Another ligand for TLR receptors on the epithelium is H pylori flagellin. This flagellin contains different amino acids than that of other bacteria in the TLR5 recognition site, as well as having a compensatory mutation that preserves bacterial motility. Those differences avoid the recognition of flagellin by TLR5[57]. H pylori also avoids recognition by TLR9, which is the receptor for unmethylated CpG motif present in bacteria and viruses. Since H pylori DNA shows a high rate of methylation, it can evade the recognition of its DNA by TLR9.
Mast cells represent another innate cell phenotype that is found within the H pylori-infected gastric mucosa of humans and mice[58]. These cells represent an innate defense component that may kill bacteria through the release of proteases and other mediators. Additionally, an interesting observation was made in a recent study that showed that these cells can mediate bacterial clearance in vaccinated mice, and were suggested to do so via a cross talk with CD4+ T cells[59].
In parallel with CagA, peptidoglycan (PGN) is also translocated into the epithelial cells by the cag pathogenicity island (PAI)-encoded type IV secretion system. Cag-PAI positive bacteria can induce the production of IL-8 via NF-κB in a manner that is CagA-independent by signaling through Nod1. Thus, H pylori PGN can interact with Nod1 and induce the activation of NF-κB[60].
CONCLUDING REMARKS
Infection with H pylori results in robust innate and acquired immune responses by the host, where the gastric epithelium represents a central player. Interaction of H pylori with the host epithelium results in the release of an array of chemokines and cytokines. Some of these factors are stimulated via the engagement of toll-like receptors or cell surface receptors, such as CD74. Also, injection of CagA via the bacterial type IV secretion system leads to NF-κB activation and the ensuing release of cytokines. The infected gastric mucosa is infiltrated by neutrophils and mononuclear cells as well as components of the acquired response, such as lymphocytes. A specific humoral response is also triggered during infection, as well as a T cell response that is skewed toward a Th1 cell response. In spite of these immune mechanisms, H pylori is not cleared because the bacteria seem to be equipped with an array of mechanisms that allows them to evade or downregulate the host responses. Understanding these multiple mechanisms is a required step toward the development of any immune intervention strategies to protect from initial infection and to eliminate infections that are already established.
S- Editor Liu Y L- Editor Alpini GD E- Editor Ma WH