Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5565
Revised: June 8, 2006
Accepted: June 16, 2006
Published online: September 14, 2006
Esophageal cancer (EC) is a highly lethal disease. Approximately 50% of patients present with metastatic EC and most patients with localized EC will have local recurrence or develop metastases, despite potentially curative local therapy. The most common sites of distant recurrence are represented by lung, liver and bone while brain and breast metastases are rare. Usually patients with advanced disease are not treated aggressively and their median survival is six months. We report a woman patient who developed breast and brain metastases after curative surgery. We treated her with a highly aggressive chemotherapeutic and surgical combination resulting in a complete remission of the disease even after 11-year follow-up. We think that in super selected patients with more than one metastasis, when functional status is good and metastases are technically resectable, a surgical excision may be considered as a salvage option and chemotherapy should be delivered to allow a systemic control.
- Citation: Santeufemia DA, Piredda G, Fadda GM, Rocca PC, Costantino S, Sanna G, Sarobba MG, Pinna MA, Putzu C, Farris A. Successful outcome after combined chemotherapeutic and surgical management in a case of esophageal cancer with breast and brain relapse. World J Gastroenterol 2006; 12(34): 5565-5568
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5565.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5565
Esophageal cancer (EC) is a highly lethal disease, with an estimated annual incidence of 14 550 new cases and 13 770 related deaths in 2006 in the USA[1]. Approximately 50% of patients present with metastatic disease and most patients with localized EC will have local recurrence or develop metastases, despite potentially curative local therapy[2].
The most common sites of distant recurrence are represented by lung, liver and bone[3] while brain and breast metastases are rare. Brain metastases have been reported only in 3.6% of all resected patients[4] but their incidence in all patients with EC is higher[5].
Extra mammary malignancies rarely metastasize to the breast, usually in patients with disseminated neoplasms[6] and according to literature data, only few cases of breast metastases from EC have been reported so far[7-9].
The case presented here is a woman with EC who underwent surgery and later developed synchronous solitary breast and brain metastases. She showed uncommon sites of recurrence of the disease. Generally, patients with EC are not treated aggressively in the presence of advanced diseases. Nevertheless, we performed a highly combined aggressive chemotherapeutic and surgical approach resulting in a complete remission of the disease even after 11-year follow-up. Together with the clinical case description, considering the rarity of the event, we discussed the features connected with the diagnosis and management of such an uncommon presentation of the metastatic disease.
In June 1995 a 51-year-old white woman patient with EC was admitted to our hospital. She complained of severe dysphagia and dyspeptic disorders associated with a weight loss. Her history revealed recent onset of dysphagia, present at first due to solid foods and then due to fluids as well. The difficult food intake caused a subsequent 5 kg weight loss during the five months prior to our observation. Since she had no other specific complaints, we decided to submit our patient to instrumental checks.
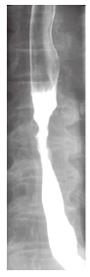
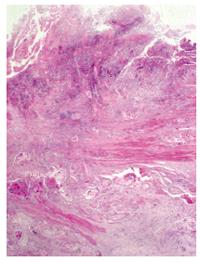
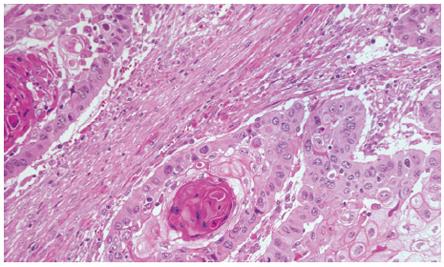
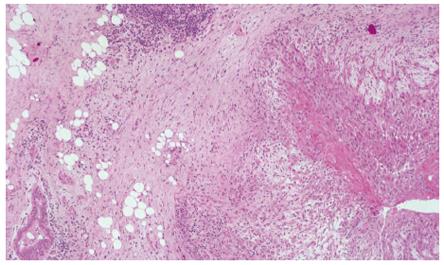
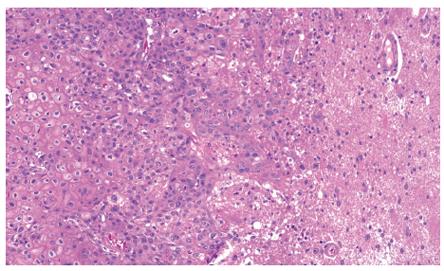
Barium swallow showed the presence of a 4 cm stenotic trait in the middle thoracic esophagus (Figure 1) and a next esophagogastroduodenoscopy revealed a vegetant mass in the esophageal lumen causing the stenosis. A biopsy of the mass was performed and histologic examination revealed a squamous esophageal carcinoma (Figures 2 and 3). The tumor marker values were increased and carbohydrate antigen 19-9 (CA19-9) was 250 U/mL (normal < 40 U/mL) while carcinoembryonic antigen (CEA) was 24 μg/L (normal < 3 μg/L). Other laboratory findings were normal.
Total body CT scan, abdominal ultrasonography and bone scintigraphy revealed that the disease was at stage IIa, we therefore decided to treat the patient surgically. In July 1995, subtotal esophagectomy was performed with dissection of lymph nodes and retrosternal reconstruction using tubulized gastric stump. Surgical operation was radical and the lymph nodes were negative for metastases. Histological examination confirmed the presence of squamous carcinoma and pathological stage of the disease was T2 No Mo. The woman underwent periodical clinical checks.
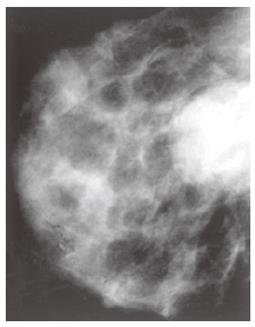
In November 1995, the patient noted the presence of an approximately 3 cm × 3 cm nodule in the upper lateral quadrant of the left breast. At clinical examination, it appeared as a hard mass not fixed to the surrounding structures. The tumor marker carbohydrate antigen 15-3 (CA15-3) was normal. A mammography confirmed the presence of the nodule (Figure 4) and fine-needle aspiration cytology under ultrasound guidance was performed, indicating the presence of squamous carcinoma cells.
Total body CT scan requested for restaging showed also a 1 cm × 1 cm focal lesion in the frontal right lobe of the brain. Therefore, we treated the woman with a palliative chemotherapy schedule based on cisplatin (25 mg/m2 iv a day) from d 1 to d 5, and 5-fluorouracil (1000 mg/m2 iv a day) from day 1 to day 5 by continuous infusion every four weeks[10]. After the first cycle administration we observed remarkable reduction of the breast metastasis at clinical examination.
Taking into account either the palliative medical treatment or her relatively young age and good general conditions, we decided to perform surgical excision of both metastases followed by chemotherapy. In the following ten days, either tumorectomy or brain metastasectomy of the breast lesion was performed. Histological examination (Figures 5 and 6) revealed the presence of esophageal metastatic tumor. No surgical complication occurred. One month later, we resumed chemotherapy and further five courses of treatment were delivered.
In the following time the patient began to start again her daily activities and underwent periodical clinical and instrumental checks, which were always negative. The woman enjoyed good health after eleven years.
EC is treatable but rarely curable. Patients with metastatic EC have a median survival time of six months[11]. Moreover the reported 5-year survival rate ranges from 20% to 36% after intentionally curative surgery[12] due to a high rate of either local or distant recurrence. Distant metastasis rate is reported to be 26% within 20 mo after radical surgery[13]. Early metastatic relapse after complete resection of any apparently localized primary tumor indicates that micrometastatic tumor cell spread at the time of surgery is undetectable by current staging methods and routine histopathology[14].
Commonly EC metastasizes to the lungs, liver and bone[3] but rarely to the brain[4] and exceptionally to the breast[7-9]. We observed an unusual metastatic pattern with both solitary breast and brain single metastases in our patient. Breast metastases from non-mammary malignancies are very rare and their incidence ranges from 0.5% to 5.1% of all breast tumors[7]. They usually occur in the upper outer quadrant[6] and their prognosis is poor[8,9].
Clinical and radiological aspects of secondary breast tumors are heterogeneous, though in most cases they appear as a solitary palpable, usually mobile mass. Mammographic finding consists of a round, dense and well-circumscribed mass, without spiculature, microcalcification or skin thickening. Furthermore, growth is usually rapid[15]. However, differential diagnosis is needed for correct treatment choice and it is reasonable to obtain a histological or cytological sample of the lesion.
The presence of metastatic lesions in any patient is an indication for a systemic treatment of primary cancer. Anyhow it has been reported that curative surgery for breast metastasis is a correct choice of treatment if metastases in other sites are controlled by other modalities[7].
For the case reported here, we made a disease restaging which showed concomitant presence of a brain metastasis because of the detection of her breast metastasis. This feature represents a further worsening of the prognosis of our patient. As a matter of fact patients with brain metastases from EC have a median survival time of 3.9 mo[16]. However it has been reported that an intensive brain tumor treatment may result in a longer survival time of selected patients with good Karnofsky performance status (KPS)[17] after excision of a single brain metastasis from EC[18]. Even the presence of multiple or recurrent brain metastases does not automatically controindicate surgery because it can prolong survival and enhance the quality of life[19]. Currently surgery followed by whole brain radiation therapy (WBRT) is considered the standard treatment[20] for a single brain metastasis.
For the case reported here, we began chemotherapy in order to obtain a systemic control of the disease. Since we observed a remarkable reduction of the breast lesion after only one course of chemotherapy, we modified our treatment plan. Since the patient was relatively young and in good functional status, we decided to perform radical excision of her metastatic EC followed by chemotherapy. Brain radiotherapy was not performed. This combined aggressive chemotherapeutic and surgical approach resulted in a complete remission of the disease even after 11-year follow-up.
Generally, the prognosis of patients with recurrent EC is poor, but in some cases surgical resection, chemotherapy or radiotherapy has been proven effective[21].
Our experience suggests that when functional status is good and metastases are technically resectable, surgical excision may be considered in selected patients with more than one metastasis and chemotherapy should be delivered to allow a systemic control.
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4370] [Cited by in RCA: 4261] [Article Influence: 213.1] [Reference Citation Analysis (0)] |
| 2. | Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol. 1999;26:12-20. [PubMed] |
| 3. | Posner MC, Forastiere AA, Minsky BD. Cancer of the esophagus. Cancer Principles & Practice of Oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins 2005; 861-909. |
| 4. | Gabrielsen TO, Eldevik OP, Orringer MB, Marshall BL. Esophageal carcinoma metastatic to the brain: clinical value and cost-effectiveness of routine enhanced head CT before esophagectomy. AJNR Am J Neuroradiol. 1995;16:1915-1921. [PubMed] |
| 5. | Mandard AM, Chasle J, Marnay J, Villedieu B, Bianco C, Roussel A, Elie H, Vernhes JC. Autopsy findings in 111 cases of esophageal cancer. Cancer. 1981;48:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977;129:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Shiraishi M, Itoh T, Furuyama K, Yamasaki S, Shimada Y, Hosotani R, Nakashima Y, Imamura M. Case of metastatic breast cancer from esophageal cancer. Dis Esophagus. 2001;14:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Nielsen M, Andersen JA, Henriksen FW, Kristensen PB, Lorentzen M, Ravn V, Schiødt T, Thorborg JV, Ornvold K. Metastases to the breast from extramammary carcinomas. Acta Pathol Microbiol Scand A. 1981;89:251-256. [PubMed] |
| 9. | Miyoshi K, Fuchimoto S, Ohsaki T, Sakata T, Takeda I, Takahashi K, Ohkawa T, Murata T, Kuwada Y. A Case of Esophageal Squamous Cell Carcinoma Metastatic to the Breast. Breast Cancer. 1999;6:59-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ajani JA, Ryan B, Rich TA, McMurtrey M, Roth JA, DeCaro L, Levin B, Mountain C. Prolonged chemotherapy for localised squamous carcinoma of the oesophagus. Eur J Cancer. 1992;28A:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Albertsson M. Chemoradiotherapy of esophageal cancer. Acta Oncol. 2002;41:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lerut T, De Leyn P, Coosemans W, Van Raemdonck D, Scheys I, LeSaffre E. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg. 1992;216:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Law SY, Fok M, Wong J. Pattern of recurrence after oesophageal resection for cancer: clinical implications. Br J Surg. 1996;83:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Hosch SB, Stoecklein NH, Pichlmeier U, Rehders A, Scheunemann P, Niendorf A, Knoefel WT, Izbicki JR. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol. 2001;19:1970-1975. [PubMed] |
| 15. | McIntosh IH, Hooper AA, Millis RR, Greening WP. Metastatic carcinoma within the breast. Clin Oncol. 1976;2:393-401. [PubMed] |
| 16. | Ogawa K, Toita T, Sueyama H, Fuwa N, Kakinohana Y, Kamata M, Adachi G, Saito A, Yoshii Y, Murayama S. Brain metastases from esophageal carcinoma: natural history, prognostic factors, and outcome. Cancer. 2002;94:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 17. | Khuntia D, Sajja R, Chidel MA, Lee SY, Rice TW, Adelstein DJ, Carlson TP, Saxton JP, Barnett GH, Suh JH. Factors associated with improved survival in patients with brain metastases from esophageal cancer: a retrospective review. Technol Cancer Res Treat. 2003;2:267-272. [PubMed] |
| 18. | Kesler KA, Hammoud ZT, Helft PR, Rieger KM, Pritz MB, Brown JW. Long-term survival after excision of a solitary esophageal cancer brain metastasis. J Thorac Cardiovasc Surg. 2006;131:497-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lang FF, Sawaya R. Surgical treatment of metastatic brain tumors. Semin Surg Oncol. 1998;14:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Fujimaki T. Surgical treatment of brain metastasis. Int J Clin Oncol. 2005;10:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Saeki H, Kawaguchi H, Araki K, Ohno S, Sugimachi K. [Treatment strategy for and clinical results in patients with recurrent esophageal cancer]. Nihon Geka Gakkai Zasshi. 1999;100:185-190. [PubMed] |
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH