Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5501
Revised: December 28, 2005
Accepted: May 23, 2006
Published online: September 14, 2006
AIM: To evaluate the dosimetry, efficacy and toxicity of intensity-modulated radiation therapy (IMRT) and concurrent chemotherapy for patients with locally advanced cervical and upper thoracic esophageal cancer.
METHODS: A retrospective study was performed on 7 patients who were definitively treated with IMRT and concurrent chemotherapy. Patients who did not receive IMRT radiation and concurrent chemotherapy were not included in this analysis. IMRT plans were evaluated to assess the tumor coverage and normal tissue avoidance. Treatment response was evaluated and toxicities were assessed.
RESULTS: Five- to nine-beam IMRT were used to deliver a total dose of 59.4-66 Gy (median: 64.8 Gy) to the primary tumor with 6-MV photons. The minimum dose received by the planning tumor volume (PTV) of the gross tumor volume boost was 91.2%-98.2% of the prescription dose (standard deviation [SD]: 3.7%-5.7%). The minimum dose received by the PTV of the clinical tumor volume was 93.8%-104.8% (SD: 4.3%-11.1%) of the prescribed dose. With a median follow-up of 15 mo (range: 3-21 mo), all 6 evaluable patients achieved complete response. Of them, 2 developed local recurrences and 2 had distant metastases, 3 survived with no evidence of disease. After treatment, 2 patients developed esophageal stricture requiring frequent dilation and 1 patient developed tracheal-esophageal fistula.
CONCLUSION: Concurrent IMRT and chemotherapy resulted in an excellent early response in patients with locally advanced cervical and upper thoracic esophageal cancer. However, local and distant recurrence and toxicity remain to be a problem. Innovative approaches are needed to improve the outcome.
- Citation: Wang SL, Liao Z, Liu H, Ajani J, Swisher S, Cox JD, Komaki R. Intensity-modulated radiation therapy with concurrent chemotherapy for locally advanced cervical and upper thoracic esophageal cancer. World J Gastroenterol 2006; 12(34): 5501-5508
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5501
Cervical esophageal cancer occurs rarely and accounts for only 2%-10% of all esophageal carcinomas in the United States[1]. Surgery, an option only for patients with early-stage tumors, generally requires a total laryngopharyngoesophagectomy with reconstruction, an operation often leads to considerable dysfunction. The 5-year overall survival rate was only 16%-28% in two studies of patients treated with curative surgery[2,3]. Unfortunately, surgical resection is not an appropriate treatment for those with locally advanced tumors, because it is difficult to achieve a clear margin. Radiotherapy alone, which does preserve laryngeal function, produces poor results, as evidenced by two studies of patients treated with definitive radiotherapy alone, showing the 5-year overall survival rate of only 14%-25%[4,5].
Concurrent chemoradiotherapy is now standard treatment for locally advanced esophageal cancer, based on the results of an intergroup randomized controlled trial (RTOG 8501)[6]. Four studies examined the results from RTOG trial among small cohorts of cervical esophageal cancer patients who were treated with concurrent chemoradiotherapy[7-10]. The 5-year survival rate was 55% in one[9], and the 10-year survival rate was 27% in another[8].
As to radiotherapy, treatment planning for cervical esophageal cancer is challenging partly because of the anatomical structures involved. The cervical esophagus lies in a close proximity to the spinal cord, courses through the lower neck and upper thorax with drastic change of contour and diameter of the anatomy; and the lymph nodes at risk must be incorporated into the irregular treatment volume.
Evidence suggested that, in these patients, high-dose radiotherapy results in better local control and survival compared with low-dose radiotherapy[11] However, high doses of radiotherapy may be associated with potentially high risk of complication because of the adjacent critical structure involved in the high radiation region, such as spinal cord.
Intensity-modulated radiation therapy (IMRT) is a novel approach to the planning and delivery of radiation therapy. Numerous investigators have demonstrated the benefits of IMRT planning in a variety of tumor sites in terms of the feasibility of normal tissue sparing[12-14] and the delivery of higher radiation doses than conventional doses[15,16]. Another advantage of IMRT is its ability to deliver differentiated dose to various structure during same fraction dose irradiation, thus allows to deliver a higher dose to gross tumor and lower dose to subclinical disease during same session of beam deliver, as commonly described as “simultaneous integrated boost (SIB)”.
In two recent studies of esophageal cancer, IMRT plans were found to be superior to three-dimensional conformal radiation therapy (3D-CRT) plans in terms of dose conformity, homogeneity, and sparing of critical normal structure, such as the spinal cord and lung[17,18]. An another study demonstrated that IMRT plans produced better dosimetric results than 3D-CRT in patients with cervical esophageal cancer[19]. However, there is no clinical treatment result reported on patients with cervical esophageal cancer who were treated with IMRT.
At the University of Texas MD Anderson Cancer Center, we have used IMRT concurrently with chemotherapy for all patients with cervical esophageal cancer and for some with upper thoracic esophageal cancer since August 2002. We performed a retrospective study to evaluate the dosimetric considerations, early response and toxicity of this group of patients.
In this retrospective study, conducted from August 1, 2002 through December 31, 2004, 7 patients were selected according to the following inclusion criteria: newly diagnosed locally advanced cervical and upper thoracic esophageal cancer, definitive concurrent IMRT with chemotherapy treatment at the University of Texas MD Anderson Cancer Center, recoverable treatment plan available. Patients were excluded if they had a distant metastasis or had esophagectomy or previously had radiotherapy in the neck or thoracic region. This study was approved by our Institutional Review Board, and informed consent for radiation therapy from all patients was taken. HIPPA compliance was enforced.
For their pretreatment evaluation, all patients underwent computed tomography (CT) of the neck, chest, and abdomen, positron emission tomography (PET)-CT, and endoscopy and biopsy of the esophagus. Five patients underwent endoscopic sonography of the esophagus; the other 2 patients did not undergo endoscopic ultrasound examination due to severe obstruction of the esophagus by tumor; instead, they underwent bronchoscopy and PET-CT, which confirmed that they had T4N0 and T4N1 disease. In total, 6 of the 7 patients underwent bronchoscopy. The 1997 American Joint Committee on Cancer guidelines for the staging of cancer were used to classify tumor stage. Tumor stage was determined by endoscopic sonography and bronchoscopy findings, nodal stage was determined by endoscopic sonography or PET-CT, and metastasis stage was determined by PET-CT.
The induction chemotherapy consisted of two cycles of weekly carboplatin and paclitaxel (n = 1), given before chemoradiation. The concurrent chemotherapy consisted of continuous infusion of 5-fluorouracil (5-FU) (1000 mg/m2) on d 1 to 4 and 29 to 32 and cisplatin (75 mg/m2) on d 1 and 29 (n = 2), continuous infusion of 5-FU (700 mg/m2) on d 1 to 5 and 29 to 33 and cisplatin (75 mg/m2) on d 1 and 29 (n = 1), continuous infusion of 5-FU (300 mg/m2) on Monday to Friday for 5 wk and paclitaxel (45-50 mg/m2) weekly (n = 2), carboplatin area under the curve (AUC) 2 twice weekly and paclitaxel (30 mg/m2) weekly for 5 wk (n = 1), or carboplatin AUC 1 once weekly and docetaxel (20 mg/m2) weekly and continuous infusion of 5-FU (200 mg/m2) on Monday to Friday for 5 wk (n = 1).
All patients underwent CT simulation in a supine position with their arms by their sides; the CT images were taken at a 3-mm thickness throughout the entire neck and thorax. Four of the patients were immobilized with a head and neck/upper thoracic thermoplastic mask, and three with a vacuum-locked cradle. The gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), spinal cord, and lung parenchyma were outlined on each image. The GTV was defined as any visible tumor on the image. The CTV was defined as the GTV plus a 2- to 5-cm margin superior to the highest extension of the tumor and a 4- to 5-cm margin inferior to the lowest extension of the tumor with a 2-cm radial margin. Uninvolved bony structure and lung tissue were kept outside the CTV. The PTV was defined as the CTV plus a 5-mm margin. For patients in whom the SIB was used, the GTV boost was defined as the initial GTV plus a 1.5-cm surrounding margin. A 5-mm margin around normal structures, such as the spinal cord and lung, was also added for the planning organ-at-risk volume (PRV).
The inverse IMRT plans for 4 of the 7 patients were created using Corvus software (v4.0) (Corvus, Nomos Inc., Sewickley, PA) and for the other 3 using Pinnacle (v6.2) software (Philips Radiation Oncology Systems, Andover, MA). All treatment plans used heterogeneity correction and were delivered with 6-MV photons. The mean dose, dose range, and standard deviation (SD) of the PTV (for GTV boost and CTV) were calculated. The minimum dose to the PTV was defined as the dose to the coldest 1% of target volume, and the maximum dose was defined as the dose to the hottest 1% of target volume. The SD percentage of the PTV was the SD dose to the PTV divided by the prescription dose to the PTV. The maximum dose to the spinal cord was defined as that received by 1 cm3 of the volume. The mean dose and the lung V20 (volume of lung receiving ≥ 20 Gy) for the total lung were calculated. The lung V20 < 40% and the maximum spinal cord dose of 45 Gy were considered to be acceptable for the IMRT plan. The pretreatment dosimetric quality assurance procedure and a test run were performed before the start of radiotherapy. Radiotherapy was delivered in a step-and-shoot mode with multi-leaf collimators. Portal films were obtained weekly to ensure the correct set-up.
Acute toxicity levels were assessed weekly with complete blood cell counts and examinations for esophagitis and skin reactions during the concurrent chemoradiotherapy. Esophageal toxicity was defined as late if it occurred more than 90 d after radiotherapy. Radiation pneumonitis was defined as acute if it occurred within 6 mo after radiotherapy. All toxic effects were assessed using RTOG criteria[20].
The following evaluations were performed 1 mo after treatment, every 3 mo for the first 2 years, and every 6 mo thereafter: physical examination, complete blood cell count, blood chemistry tests, an endoscopic examination, an esophageal biopsy, and scans of the neck, chest, and abdomen by CT, PET-CT, or both. Endoscopic sonography of the esophagus was performed in two of the patients to evaluate lymph node response.
A complete response (CR) of the primary tumor was defined as: (1) endoscopic examination was negative for all visible tumors and biopsy was negative for tumor cells lasted for ≥ 4 wk, and (2) no evidence of abnormal hypermetabolism on PET-CT scan. The response of the metastatic lymph nodes was assessed by CT, PET-CT, or endoscopic sonography of the esophagus. A CR was defined as the complete disappearance of all measurable and assessable disease for ≥ 4 wk.
The survival was calculated from the date of diagnosis.
The median time of follow-up was 15 mo (range: 3-21 mo). Of the 7 patients included in this study, 6 were men. The Karnofsky performance status scores ranged from 70 to 90, and their ages ranged from 52 to 78 years. Six patients had cervical esophageal squamous cell carcinoma, and 1 patient had upper thoracic esophageal adenocarcinoma. At the time of presentation, 2 patients tolerated solid foods, 2 tolerated soft foods, and 3 tolerated only liquid diet. Three patients had lost more than 10% of their weight in the 3 mo before treatment.
Patients had locally advanced disease as follows: 3 patients had biopsy-proven tracheal invasion by tumor through bronchoscopy examination, rendered them as T4 stage; one of them had 60% tracheal obstruction with a tracheal-esophageal (TE) fistula and had a tracheal stent placement before chemoradiation treatment; 3 patients had T3 and 1 had T2N1 stage tumors. According to the endoscopic and PET-CT findings, the tumor length ranged from 3 to 8 cm. In 6 patients, a percutaneous gastrostomy or jejunostomy feeding tube was placed prior to the start of radiotherapy because of severe dysphagia, poor nutritional status, or a high risk of aspiration. The characteristics of the patients, tumor, and treatment are summarized in Table 1.
| PatientNo. | Tumordistancefrom UES (cm) | Stage | Inductionchemotherapy | Concurrentchemotherapy | XRT dose(GTV) | XRT dose(CTV) | XRT dose(supraclavical regions) |
| 1 | 2 | T3N1M0 | 5-FU, CDDP | 64.8 Gy (2.31 Gy/f) | 50.4 Gy (1.8 Gy/f) | 50.4 Gy (1.8 Gy/f) | |
| 2 | 0 | T2N1M0 | 5-FU, paclitaxel | 59.9 Gy, (2.14 Gy/f) | 50.4 Gy (1.8 Gy/f) | 0 | |
| 3 | 0 | T3N1M0 | 5-FU, CDDP | 64.8 Gy (1.8 Gy/f × 8, 2.21 Gy × 21) | 51.7 Gy (1.53 Gy/f × 8, 1.88 Gy × 21) | 46 Gy (1.36 Gy/f × 8, 1.67 Gy × 21) | |
| 4 | 6 | T3N0M0 | 5-FU, paclitaxel | 59.4 Gy (1.8 Gy/f) | 59.4 Gy (1.8 Gy/f) | 0 | |
| 5 | 0 | T4N1M0 | 5-FU, CBP, docetaxel | 66 Gy (2.0 Gy/f) | 66 Gy (2.0 Gy/f) | 0 | |
| 6 | 2.5 | T4N1M0 | CBP, paclitaxel | CBP, paclitaxel | 66 Gy (2.0 Gy/f) | 56 Gy (1.7 Gy/f) | 56 Gy (1.7 Gy/f) |
| 7 | 0 | T4N0M0 | 5-FU, CDDP | 66 Gy (2.0 Gy/f) | 60 Gy (1.8 Gy/f) | 60 Gy (1.8 Gy/f) |
The primary tumor was irradiated with a total dose of 59.4-66 Gy given in 1.8-2.31 Gy/fraction. Three patients with cervical esophageal cancer also received prophylactic irradiation to the bilateral supraclavicular regions to a total dose of 46-60 Gy at 1.36-1.8 Gy/fraction. One patient with cervical esophageal cancer received bilateral supraclavicular and neck irradiation to a total dose of 56 Gy at 1.7 Gy/fraction because of small lymph node metastasis in the bilateral lower neck diagnosed by PET-CT. Three patients with cervical esophageal cancer also received irradiation to the superior mediastinum to a total dose of 56-66 Gy at 1.7-2.0 Gy/fraction. The SIB technique was used in 5 of the 6 patients with cervical esophageal cancer. In the other 2 patients, the GTV and CTV received the same fraction dose and total dose, since lymph node region was not prophylactically treated. Table 1 also shows the radiation treatment characteristics. Five, seven, eight, or nine coplanar beams with different gantry angles were used in the 7 patients. The total number of beam segments and monitor units (MU) was 218-559 and 21 252-40 812 for Corvus plans, and 198-417 and 27 423-42 504 for Pinnacle plans, respectively (Table 2). The daily treatment time was about 10-20 min.
| Number of beams | Gantry angles | Number of segments | Number of monitor units per fraction | Total fraction | Total monitorunits |
| 7 | 0, 25, 65, 141, 212, 295, 335 | 315 | 1395 | 28 | 39 060 |
| 5 | 40, 70, 220, 240, 290 | 218 | 759 | 28 | 21 252 |
| 9 | 0, 18, 35, 70, 150, 225, 295, 320, 340 | 559 | 1248 and 1468 | 8 + 21 | 40 812 |
| 7 | 0, 30, 60, 105, 260, 300, 330 | 270 | 749 | 33 | 24 717 |
| 5 | 0, 50, 120, 240, 300 | 198 | 831 | 33 | 27 423 |
| 8 | 12, 36, 60, 135, 225, 300, 324, 348 | 332 | 1288 | 33 | 42 504 |
| 9 | 0, 40, 70, 130, 160, 200, 230, 290, 320 | 417 | 972 | 33 | 32 076 |
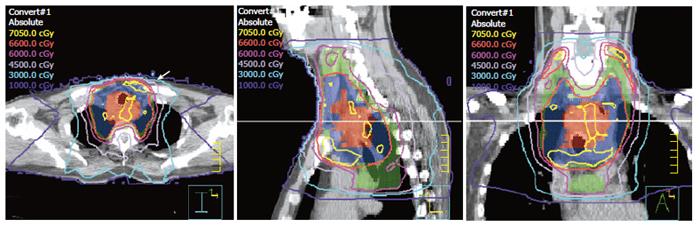
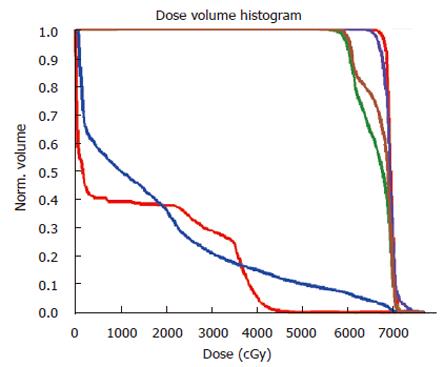
Table 3 summarizes the dosimetric parameters of IMRT plans for the 7 patients. The median GTV was 78 cm3 (range: 17-229 cm3). The median GTV dose was 64.8 Gy (range: 59.4-66 Gy), and the median CTV dose was 56 Gy (range: 50.4-66 Gy). The minimum dose received by the PTV of boost GTV ranged from 91.2 to 98.2% (SD: 3.7%-5.7%) of the prescribed dose. The minimum dose received by the PTV of CTV ranged from 93.8% to 104.8% (SD: 4.3%-11.1%) of the prescribed dose. The V20 to the bilateral lung was 10.3%-36.0% in all patients, and the lung mean dose was 6.6-17.6 Gy (median: 12.6 Gy). The maximum dose to the spinal cord ranged from 37.2 to 45.8 Gy. The dose distribution and dose volume histogram (DVH) for patient number 7 are shown in Figures 1 and 2 using pinnacle planning system.
| Patient No. | Dose (Gy) | GTV volume(cm3) | PTV meandose (Gy) | PTV min% | PTV max% | PTV SD% | Lung V20(Gy) | Lung meandose (Gy) | Spinal cordmax (Gy) |
| 1 | 64.8 (GTV) | 58 | 70.5 | 96.5 | 117.9 | 4.9 | 18.9 | 12.0 | 42.8 |
| 50.4 (CTV) | 66.4 | 104.8 | 149.6 | 11.1 | |||||
| 50.4 (sup) | 61.3 | 101.6 | 149.2 | 11.9 | |||||
| 2 | 59.9 (GTV) | 53 | 65.0 | 91.2 | 122.9 | 6.0 | 28.6 | 13.4 | 25.7 |
| 50.4 (CTV) | 58.7 | 103.9 | 139.4 | 6.6 | |||||
| 3 | 64.8 (GTV) | 85 | 68.6 | 97.8 | 117.5 | 3.7 | 10.3 | 6.6 | 37.2 |
| 51.7 (CTV) | 62.3 | 93.8 | 121.2 | 9.2 | |||||
| 46 (sup) | 54.2 | 100 | 143 | 8.1 | |||||
| 4 | 59.4 (GTV) | 17 | 65.0 | 97.1 | 119.1 | 4.3 | 24.2 | 11.6 | 39.2 |
| 5 | 66 (GTV) | 78 | 67.3 | 97.9 | 109.2 | 4.9 | 26.8 | 12.6 | 45.8 |
| 6 | 66 (GTV) | 229 | 68.0 | 98.2 | 110.9 | 4.8 | 21.9 | 7.7 | 40.2 |
| 56 (CTV + neck) | 59.8 | 101.1 | 122.9 | 9.4 | |||||
| 7 | 66 (GTC) | 169 | 66.4 | 99.4 | 111.8 | 5.7 | 36 | 17.6 | 41.8 |
| 60 (CTV) | 65.7 | 97 | 121.6 | 6.7 | |||||
| 60 (sup) | 67.1 | 105 | 116.8 | 5.4 |
One patient with cervical esophageal cancer, whose general condition gradually deteriorated, experienced excessive mucus production, weight loss and fever, and decided to begin home hospice care after 2 wk of radiotherapy, died of disease 4 mo after diagnosis. Thus, this patient’s response was not evaluable and was not included in this analysis.
The other 6 patients all achieved a CR in the primary tumor and lymph node areas after concurrent chemoradiotherapy. The response was assessed by endoscopic biopsy for all patients, 4 of them also had PET-CT. Two patients had no evidence of disease (NED) at 13 and 17 mo. Two patients developed local recurrence in the esophagus 4 and 6 mo after treatment, one of them was successfully treated with salvage photodynamic therapy and had NED at 21 mo and the other died of disease at 15 mo. One patient had lung metastasis 7 mo after treatment and was alive with disease at 17 mo. One patient had both lymph nodes recurrence in the neck and soft tissue metastasis in the left thigh 11 mo after treatment and died of disease. Table 4 shows the tumor response and failure.
| Patient No. | Response (primary and node) | Relapse | Life status | Leucopenia (grade) | Dermatitis (grade) | Esophagitis (grade) | Pneumonitis(grade) | Late esophageal toxicity (grade) |
| 1 | CR | LR | DOD | 1 | 1 | 2 | 0 | 0 |
| 2 | Not evaluable | Not evaluable | DOD | 1 | 3 | 2 | 0 | not evaluable |
| 3 | CR | NED | Alive NED | 0 | 1 | 2 | 0 | 0 |
| 4 | CR | LR | Alive NED | 3 | 1 | 1 | 0 | 3 |
| 5 | CR | NED | Alive NED | 0 | 3 | 1 | 0 | 3 |
| 6 | CR | DM and node | DOD | 0 | 3 | 2 | 0 | 0 |
| 7 | CR | DM | Alive with disease | 4 | 2 | 4 | 0 | 4 |
Acute major toxic effects included myelosuppression, dermatitis, and esophagitis. Myelosuppression occurred in 2 patients: 1 had grade 3 and another had grade 4 leukopenia. Three of the patients who had been immobilized with a thermoplastic mask experienced grade 3 skin reactions in the neck, and 1 of these required a 3-d treatment interruption. The patient who had a T4 tumor with a tracheoesophageal (TE) fistula and tracheal stent placement before chemoradiation developed grade 4 esophagitis 1 mo after treatment and had an esophageal stent placed 4 mo after treatment. Another patient experienced grade 4 late esophageal toxicity (TE fistula) 7 mo after radiotherapy and had an esophageal stent placed. Two patients experienced grade 3 late esophageal toxicity (benign esophageal strictures requiring esophageal dilatation one to four times) 4 mo and 10 mo after radiotherapy. No patient had symptomatic radiation pneumonitis, although 2 patients had radiographic changes in their irradiated lung. No patients lost more than 10% of their weight during chemoradiotherapy. No patient experienced radiation myelitis. All except 1 patient completed radiotherapy without interruption in 37-44 d. Radiotherapy was interrupted for three day in 1 patient due to grade 3 skin toxicity and radiation was completed in 50 d. Table 4 summarizes the toxicity of chemoradiotherapy.
No consensus has been reached as to the optimal radiation technique and target volume delineation for treating cervical esophageal cancer, and a survey conducted in Canada has come up with different opinions from radiation oncologists[21,22]. IMRT has potential benefit for treating cervical esophageal cancer because of the complexity of anatomy in this region. Separate IMRT plans designed for the initial large-field treatment and the subsequent boost treatment are referred as two-phase IMRT strategies. On the other hand, the term “simultaneous integrated boost” (SIB) is used to define treatment plan that delivers differentiated doses to different targets during a single fraction dose delivery as follows: one IMRT plan simultaneously delivers different dose levels to different targets in a single treatment session, which results in the primary target (e.g., palpable or visible disease or GTV) and the secondary target (e.g., regions at risk for microscopic disease or CTV) being treated to different dose levels in each and same treatment fraction. Using this approach, the fractional dose delivered to gross tumor can be increased, while, at the same time, the radiation doses and dose schedules known to be adequate for tumor control in marginal tissues and clinically uninvolved lymph nodes are preserved. Thus, the SIB-IMRT has been considered a novel method for accelerated fractionation therapy in controlling gross disease through dose-per-fraction escalation. It can shorten overall treatment time, which is preferable for treating tumors with rapid repopulation. It has the ability to deliver large IMRT fields, often required for simultaneous treatments of all target volumes, by splitting them and dynamically feathering their junctions for greater accuracy[23]. Therefore, it is an easy, efficient, and less error-prone method of planning and delivery, because it allows the same plan to be used for the entire course of treatment.
Since the large-field and boost doses are delivered in the same number of fractions, one must consider the radiobiological consequences of different fraction sizes for the gross disease, regions of microscopic spread, and electively treated lymph nodes. One can select the conventional 2 Gy per fraction for the gross disease for an SIB strategy, but that might lead to a significantly lower dose per fraction to volumes of microscopic disease and electively treated lymph nodes. On the other hand, one can choose to deliver 2 Gy per fraction to the lower and intermediate dose volumes, but this would require a high dose per fraction, as much as 2.5 Gy or more per fraction, to the gross disease. The latter scheme may have the advantage of shortening the treatment duration and a potential for improvement in local control but at an increased risk of injury to the embedded normal tissues.
In our study, the IMRT plans showed good homogeneity and conformity and the treatment time was acceptable. SIB-IMRT was used in 5 of 7 patients, which made the treatment course simple. Several researchers reported that SIB-IMRT is superior to two-phase IMRT (sequential IMRT) in terms of conformity of dose distribution within the target volume and the sparing normal tissue for head and neck cancer[24,25], lung[25,26] and prostate cancers[25]. The SIB-IMRT also has some advantage over 3-dimensional conformal radiotherapy regarding homogeneity of tumor dose and reduction of the dose to normal tissue for cervical esophageal cancer[19] and malignant glioma[27,28].
Defining target volume delineation for IMRT plan, especially a consistent CTV delineation, is challenging because current imaging techniques are not capable of directly detecting subclinical tumor involvement and also because the patterns of failure is not clear. It is difficult to differentiate if the local tumor recurrence is due to a geographic miss or persistent disease. The tendency for esophageal cancer to be multicentric or to present with submucosal skip metastasis supports the use of generous proximal and distal margins for treatment. In the RTOG 8501 trial[6], the entire esophagus was included in the radiation portals, but the toxic effects were severe. In the subsequent RTOG 9405 study[29], 5-cm proximal and distal margins and a 2-cm radial margin were added around the GTV which is effective and has been accepted as a standard in most institutions in the United States.
We used slightly smaller proximal than distal margins in our study, because of our concern about increased toxic effects on the pharynx. Prophylactic irradiation of the supraclavicular and superior mediastinal nodes could decrease the risk of nodal relapse without greatly increasing the toxic effects. In the RTOG 94-05 study[29], boost GTV was defined as a 2-cm margin around the initial GTV; we used the same boost GTV expansion in our study (1.5-cm margin from GTV to boost GTV and another 5 mm added for PTV).
This is the first study that we are aware of that reported the clinical result for esophageal cancer patients treated with IMRT. In this study, all 6 evaluable patients achieved complete response both in the primary tumor and lymph node sites. Although the early response result was very encouraging for this small group of locally advanced tumors, 2 patients developed local recurrences and another 2 had distant metastases. Only 3 patients survived with no evidence of disease during a median time of 13 mo follow-up. Although some clinical data on cervical and upper thoracic esophageal cancer patients who were treated with concurrent chemotherapy and radiotherapy with conventional or 3-dimensional conformal techniques have been published, the data are sparse because the disease is rare and the published series have consisted of relatively few patients. For example, Ampil et al[7] reported that only 2 of their 6 patients achieved CR when conventional radiation techniques were used to deliver a total dose ranging from 36 to 64 Gy at 2.0 Gy/fraction. Burmeister et al[9] reported that 31 of their 34 patients had CR after a total dose delivered by 3-dimensional conformal radiaton that ranged from 50.4 Gy in 20 fractions to 65 Gy in 33 fractions. The 5-year survival rate in this group was 55%. However, only 3 patients in this group had locally advanced disease; most had early disease. Moreover, Bidoli et al[8] reported that 37 of their 58 patients (29 of whom had cervical esophageal cancer) experienced a CR after having been given 50 Gy in 25 fractions with conventional radiation techniques. The 10-year survival rate was 27% for the 29 patients with cervical esophageal cancer. We await the maturity of our study to report the long-term outcome.
Three patients experienced severe skin toxicity, probably caused by the use of the mask and the lack of effort to avoid irradiating the skin during IMRT planning. In a study by Burmeister et al[9] with 3-dimensional conformal radiotherapy, a moderate-to-severe skin reaction occurred in most patients on the neck. IMRT technique has the potential to reduce skin toxicity if skin was defined as an avoidance structure. From our experience, to reduce toxic effects to the skin, we need to use a cradle instead of a mask to immobilize patient, also consider skin as an avoidance structure during IMRT planning.
The development of acute esophagitis during and after radiotherapy is usually unavoidable. Fortunately, no patient lost more than 10% of body weight or had to have treatment interrupted because of esophagitis, probably because of the intensive supportive and nutritional therapy received through a gastrostomy or jejunostomy tube. Therefore, the prophylactic use of such tubes is recommended for patients who have severe dysphagia or poor nutritional status prior to the initiation of chemoradiation.
Development of late esophageal toxic effects is a concern for esophageal cancer survivors. An esophageal stricture caused by fibrosis of the esophagus, usually at the site of tumor, was mainly due to the high total radiation dose delivered[30]. In this study, 3 of 4 surviving patients had esophageal stricture or fistula. Burmeister et al[9] demonstrated that in 34 patients treated with 3-dimensional conformal radiotherapy, strictures were the most notable late effect of the therapy: 11 patients developed mild strictures that did not require dilation, 4 patients developed strictures requiring repeated dilation, and 1 patient developed a severe stricture that did not respond to dilation. In the patient with a severe stricture, the attempted corrective surgery failed and resulted in death of the patient. In addition, the local tumor recurrence remains to be a concern even though relatively high radiation dose was used for this group of patients. The degree of dose escalation by IMRT was limited by long-term toxicity, such as esophageal strictures and fistula. For patients who had their local diseases well-controlled, development of distant metastases became a major pattern of failure.
Therefore, new approaches besides IMRT are needed. One possible way is to use induction chemotherapy. Theoretically, induction chemotherapy could treat the occult micrometastatic disease upfront and may lead to a decrease in the incidence of distant metastases; in addition, induction chemotherapy could be effective in shrinking the primary tumor so that only moderate radiation dose is required to control the local disease, while reducing the late esophageal toxicity. Stuschke et al[10] reported their results of induction chemotherapy plus concurrent chemoradiotherapy (a total dose of 60 to 65 Gy in 6 wk delivered by 3D-CRT) for 22 patients with upper and midthoracic locally advanced squamous cell carcinomas. Locoregional recurrences as one site of first relapse were observed in 12 patients and distant metastases as one site of first relapse occurred in 4 patients. Seven long-term survivors had a good swallowing function. Induction chemotherapy results in a major response rate of 45% and response to induction chemotherapy is strongly associated with long-term survival and locoregional tumor control. No long-term survivors are found among non-responders to induction chemotherapy. The criterion good response to induction chemotherapy can be used to select patients for high-dose radiotherapy. Another way is molecular targeting agents. Epidermal growth factor inhibitor (EGFR), such as cetuximab, in combination with radiotherapy yielded positive results in treating head and neck squamous cell carcinoma[31]. It is perhaps worthwhile to test EGFR in treatment of cervical and upper thoracic esophageal cancer, because squamous cell carcinoma is the major histology.
In conclusion, concurrent IMRT and chemotherapy resulted in an encouraging tumor response in patients with locally advanced cervical and upper thoracic esophageal cancer. However, local and distant recurrence, and late toxicity remain to be a challenge in the management of this disease. Additional innovative treatment modalities are needed to improve the outcome. All patients with cervical and upper esophageal tumor should be registered and treated in a prospective study with consistent chemotherapy and IMRT dose schedule, and possibly target therapy.
| 1. | Lee DJ, Harris A, Gillette A, Munoz L, Kashima H. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J. 1984;77:1365-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Laterza E, Mosciaro O, Urso US, Inaspettato G, Cordiano C. Primary carcinoma of the hypopharynx and cervical esophagus: evolution of surgical therapy. Hepatogastroenterology. 1994;41:278-282. [PubMed] |
| 3. | Kelley DJ, Wolf R, Shaha AR, Spiro RH, Bains MS, Kraus DH, Shah JP. Impact of clinicopathologic parameters on patient survival in carcinoma of the cervical esophagus. Am J Surg. 1995;170:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Mendenhall WM, Parsons JT, Vogel SB, Cassisi NJ, Million RR. Carcinoma of the cervical esophagus treated with radiation therapy. Laryngoscope. 1988;98:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Pearson JG. The present status and future potential of radiotherapy in the management of esophageal cancer. Cancer. 1977;39:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1396] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 7. | Ampil FL, Mills GM, Burton GV. Induction chemotherapy followed by concomitant chemoradiation for cervical esophageal cancer. Am J Gastroenterol. 1999;94:2325-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bidoli P, Bajetta E, Stani SC, De CD, Santoro A, Valente M, Zucali R, Valagussa P, Ravasi G, Bonadonna G. Ten-year survival with chemotherapy and radiotherapy in patients with squamous cell carcinoma of the esophagus. Cancer. 2002;94:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Burmeister BH, Dickie G, Smithers BM, Hodge R, Morton K. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg. 2000;126:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Stuschke M, Stahl M, Wilke H, Walz MK, Oldenburg AR, Stüben G, Jahnke K, Seeber S, Sack H. Induction chemotherapy followed by concurrent chemotherapy and high-dose radiotherapy for locally advanced squamous cell carcinoma of the cervical oesophagus. Oncology. 1999;57:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mendenhall WM, Million RR, Bova FJ. Carcinoma of the cervical esophagus treated with radiation therapy using a four-field box technique. Int J Radiat Oncol Biol Phys. 1982;8:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Uy NW, Woo SY, Teh BS, Mai WY, Carpenter LS, Chiu JK, Lu HH, Gildenberg P, Trask T, Grant WH. Intensity-modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Vicini FA, Sharpe M, Kestin L, Martinez A, Mitchell CK, Wallace MF, Matter R, Wong J. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, Amols H, Venkatraman ES, Leibel SA. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 548] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Wu VW, Sham JS, Kwong DL. Inverse planning in three-dimensional conformal and intensity-modulated radiotherapy of mid-thoracic oesophageal cancer. Br J Radiol. 2004;77:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Nutting CM, Bedford JL, Cosgrove VP, Tait DM, Dearnaley DP, Webb S. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiother Oncol. 2001;61:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Fu WH, Wang LH, Zhou ZM, Dai JR, Hu YM, Zhao LJ. Comparison of conformal and intensity-modulated techniques for simultaneous integrated boost radiotherapy of upper esophageal carcinoma. World J Gastroenterol. 2004;10:1098-1102. [PubMed] |
| 20. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3592] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 21. | Tai P, Van Dyk J, Yu E, Battista J, Schmid M, Stitt L, Tonita J, Coad T. Radiation treatment for cervical esophagus: patterns of practice study in Canada, 1996. Int J Radiat Oncol Biol Phys. 2000;47:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Tai P, Van Dyk J, Yu E, Battista J, Stitt L, Coad T. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;42:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Wu Q, Arnfield M, Tong S, Wu Y, Mohan R. Dynamic splitting of large intensity-modulated fields. Phys Med Biol. 2000;45:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys. 2000;46:619-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Dogan N, King S, Emami B, Mohideen N, Mirkovic N, Leybovich LB, Sethi A. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phys. 2003;57:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Dirkx ML, van Sörnsen De Koste JR, Senan S. A treatment planning study evaluating a 'simultaneous integrated boost' technique for accelerated radiotherapy of stage III non-small cell lung cancer. Lung Cancer. 2004;45:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Chan MF, Schupak K, Burman C, Chui CS, Ling CC. Comparison of intensity-modulated radiotherapy with three-dimensional conformal radiation therapy planning for glioblastoma multiforme. Med Dosim. 2003;28:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Thilmann C, Zabel A, Grosser KH, Hoess A, Wannenmacher M, Debus J. Intensity-modulated radiotherapy with an integrated boost to the macroscopic tumor volume in the treatment of high-grade gliomas. Int J Cancer. 2001;96:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 418] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Sykes AJ, Burt PA, Slevin NJ, Stout R, Marrs JE. Radical radiotherapy for carcinoma of the oesophagus: an effective alternative to surgery. Radiother Oncol. 1998;48:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3919] [Cited by in RCA: 3708] [Article Influence: 185.4] [Reference Citation Analysis (0)] |
S- Editor Pan BR L- Editor Kumar M E- Editor Bai SH