Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5490
Revised: October 28, 2005
Accepted: March 10, 2006
Published online: September 14, 2006
AIM: To determine circulating and hepatic adiponectin in rodents with fatty liver disease or liver cirrhosis and investigate expression of the adiponectin receptors AdipoR1 on the mRNA and protein level and AdipoR2 on the mRNA level.
METHODS: Fat fed rats were used as a model for fatty liver disease and bile duct ligation in mice to investigate cirrhotic liver. Expression of AdipoR1 and AdipoR2 mRNA was determined by real time RT-PCR. AdipoR1 protein was analysed by immunoblot. Adiponectin was measured by ELISA.
RESULTS: Systemic adiponectin is reduced in fat-fed rats but is elevated in mice after bile duct ligation (BDL). Hepatic adiponectin protein is lower in steatotic liver but not in the liver of BDL-mice when compared to controls. Adiponectin mRNA was not detected in human liver samples or primary human hepatocytes nor in rat liver but recombinant adiponectin is taken up by isolated hepatocytes in-vitro. AdipoR1 mRNA and AdipoR1 protein levels are similar in the liver tissue of control and fat fed animals whereas AdipoR2 mRNA is induced. AdipoR2 mRNA and AdipoR1 mRNA and protein is suppressed in the liver of BDL-mice.
CONCLUSION: Our studies show reduced circulating adiponectin in a rat model of fatty liver disease whereas circulating adiponectin is elevated in a mouse model of cirrhosis and similar findings have been described in humans. Diminished hepatic expression of adiponectin receptors was only found in liver cirrhosis.
- Citation: Neumeier M, Hellerbrand C, Gäbele E, Buettner R, Bollheimer C, Weigert J, Schäffler A, Weiss TS, Lichtenauer M, Schölmerich J, Buechler C. Adiponectin and its receptors in rodent models of fatty liver disease and liver cirrhosis. World J Gastroenterol 2006; 12(34): 5490-5494
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5490
Obesity and especially visceral fat accumulation cause insulin resistance, a common risk factor for hepatic steatosis. Fatty liver is thought to represent the first step towards the subsequent development of liver fibrosis. Impaired mitochondrial function provides the second hit and promotes the generation of reactive oxygen species, which promote lipid peroxidation, the release of inflammatory cytokines, death of hepatocytes and activation of hepatic stellate cells. Non-alcoholic steatohepatitis (NASH) is a progressive disorder that can lead to liver cirrhosis and even hepatocellular carcinoma[1].
Adiponectin is highly abundant in human serum and is secreted by adipose tissue in inverse proportion to the body mass index[2]. Adiponectin improves whole body insulin sensitivity and in addition exerts anti-inflammatory effects by reducing NFκB activation[3]. Low adiponectin levels are associated with NASH independent of insulin resistance and body mass index indicating a protective effect for adiponectin in liver disease[4]. This idea is supported by studies in rodents where recombinant adiponectin given to leptin-deficient ob/ob mice ameliorates hepatic steatosis and normalizes alanine aminotransferase levels[5]. Besides these protective effects on fatty liver disease adiponectin attenuates T-cell mediated hepatic inflammation by reducing the release of proinflammatory cytokines, the activation of hepatic stellate cells and cell death of hepatocytes[6].
Two 7-transmembrane proteins, AdipoR1 and AdipoR2, have been identified to function as adiponectin receptors[7]. AdipoR1 mRNA is mainly expressed in the human heart and skeletal muscle, whereas AdipoR2 was supposed to be the main receptor in the liver[7]. Recently a prominent protein expression of AdipoR1 in primary hepatocytes was demonstrated indicating that AdipoR1 may also be important in hepatic signal transduction[8]. Adiponectin activates the AMP-activated protein kinase (AMPK) and PPARα[7] but may also inhibit the binding of growth factors to their corresponding receptors independent of AdipoR1 and AdipoR2[9].
Although there is a well documented relationship between low adiponectin and liver disease, the role of adiponectin receptors is less clear[4,6]. In addition, mainly AdipoR2 has been analysed with regard to liver function[10,11]. Therefore the expression of AdipoR1 was investigated in rodent models of fatty liver disease and liver cirrhosis.
Wistar rats were fed a standard rodent chow (6 animals) or a high fat diet for twelve weeks (six animals) as recently described[12]. Male C57Bl/6J mice (body weight 25-30 g) underwent common bile duct ligation (BDL) and transection as previously reported[13]. Another group of animals was sham-operated to serve as a control. For analysis of adiponectin or AdipoR1 protein four control and five BDL mice were used. Analysis of mRNA expression was performed with total RNA isolated from the liver of eight control and four BDL animals. All animal procedures were performed under the guidelines set by The University of Regensburg Institutional Animal Care and Use Committee. Primary hepatocytes from three different donors were isolated and cultivated as described before[14]. Tissue samples from human liver resection were obtained from patients undergoing partial hepatectomy. Experimental procedures were performed according to the guidelines of the charitable state controlled foundation HTCR (Human Tissue and Cell Research), with the informed patient’s consent[15] approved by the local ethical committee of the University of Regensburg.
RPMI medium was from Biochrom (Southborough, MA, USA), RNeasy Mini Kit from Qiagen (Hilden, Germany) and oligonucleotides were synthesized by Metabion (Planegg-Martinsried, Germany). LightCycler FastStart DNA Master SYBR Green I was purchased from Roche (Mannheim, Germany). AdipoR1 peptide antibody was raised as recently described[8]. AdipoR2 protein was not analysed because several antibodies investigated did not specifically detect in-vitro translated AdipoR2 by immunoblot (own unpublished results).
ELISAs for human and mouse adiponectin and human recombinant adiponectin were from R&D systems (Wiesbaden-Nordenstadt, Germany). ELISAs for rat adiponectin from BioCat (Heidelberg, Germany). PI3-Kinase p85 antibody was from Upstate (Lake Placid, NY, USA). Recombinant human leptin was from Sigma Chemical (Deisenhofen, Germany) and 100 μg/L were used.
Real-time RT-PCR was performed as recently described[16]. The primers are listed in Table 1. Amplification in the LightCycler capillaries was done for 45 cycles with initial incubation of ten minutes at 95°C for activation of Taq polymerase. Cycling parameters were 15 s at 95°C, ten seconds 60°C and ten seconds at 72°C. The second derivative method was used for quantification with the LightCycler software. For quantification of the results the standard curve method was used. Normalization was performed by dividing each value calculated for a specific gene by the value of the corresponding housekeeping gene. QuantiTect Primer Assays (Qiagen) for use in real-time RT-PCR with SYBR Green detection were used to determine rat adiponectin mRNA.
| Name | Sequence |
| AdipoR1 uni (h) | 5´-GGGGAATTCTCTTCCCACAAAGGATCTGTGGTG-3´ |
| AdipoR1 rev (h) | 5´-GGGCTGCAGTTAAGTTTCTGTATGAATGCGGAAGAT-3´ |
| AdipoR2 uni (h, m) | 5´-GGGGAATTCAACGAGCCAACAGAAAACCGATTG-3´ |
| AdipoR2 rev (h, m) | 5´-GGGCTGCAGCTAAATGTTGCCTGTTTCTGTGTGTAT-3´ |
| β-actin uni (h) | 5'-CTACGTCGCCCTGGACTTCGAGC-3´ |
| β-actin rev (h) | 5'-GATGGAGCCGCCGATCCACACGG-3´ |
| AdipoR1 uni (m) | 5´-AGGCCTGTCCACCATCAC-3´ |
| AdipoR1 rev (m) | 5´-CAGAAGGAGCCCCATTGC-3´ |
| AdipoR1 uni (r) | 5´-CGACAGGCCTAAGTGTCCAT-3´ |
| AdipoR1 rev (r) | 5´-CTTACCCTTCTCCTCCAGCA-3´ |
| AdipoR2 uni (r) | 5´-GAAGGAGGGTCAACTCACCA-3´ |
| AdipoR2 rev (r) | 5´-CATCAAGTTGGTGCCCTTTT-3´ |
| β-actin uni (m) | 5'-TGGAATCCTGTGGCATCCATG-3´ |
| β-actin rev (m) | 5'-TAAAACGCAGCTCAGTAACAG-3´ |
| adiponectin (h) | 5´-CATGACCAGGAAACCACGACT´-3´ |
| adiponectin (h) | 5´-TGAATGCTGAGCGGTAT-3´ |
The cells or tissues were solubilized in RIPA buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis and were transferred to PVDF membranes (Bio-Rad, Germany). Incubations with antibodies were performed in 1% BSA in PBS, 0.1% Tween overnight. Detection of the immune complexes was carried out with the ECL Western blot detection system (Amersham Pharmacia, Deisenhofen, Germany).
Data are represented as Box Plots (Sigma Plot) indicating the median, the upper and lower quartile, the largest and the lowest value in the data set. Statistical differences were analysed by Student´s t-test (MS Excel) and a value of P < 0.05 was regarded as statistically significant.
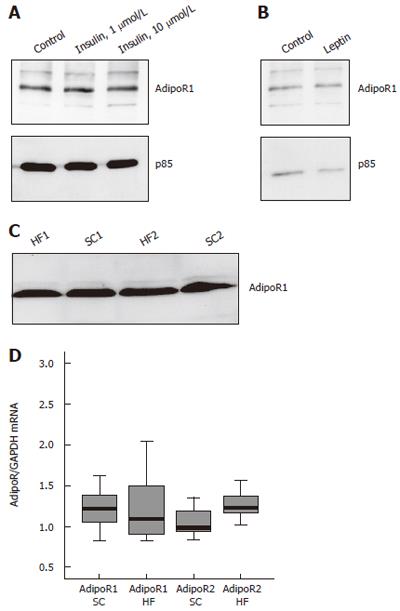
Expression of AdipoR1 was analysed in HepG2 cells treated with insulin or leptin. HepG2 cells were treated with insulin, 1 μmol/L and 10 μmol/L for four hours (Figure 1A) and six hours (not shown) or leptin for 24 h. AdipoR1 was analyzed by immunoblot and was found not to be regulated by insulin or leptin (Figures 1A and 1B). AdipoR1 and AdipoR2 mRNA expression was determined in insulin treated cells and was similar in controls and insulin-incubated HepG2 cells (not shown).
AdipoR1 protein and mRNA as well as AdipoR2 mRNA were analysed in the liver of rats on a standard chow (SC) or on a high fat (HF) diet. AdipoR1 protein level is similar in these animals (Figure 1C) and real-time RT-PCR revealed no alterations in the mRNA expression of AdipoR1 whereas AdipoR2 mRNA is induced with a relative expression of 1.0 ± 0.2 in SC and 1.3 ± 0.2 in the HF group (P = 0.004) (Figure 1D). RT-PCR data were normalized by the corresponding GAPDH values which were not different between the two groups.
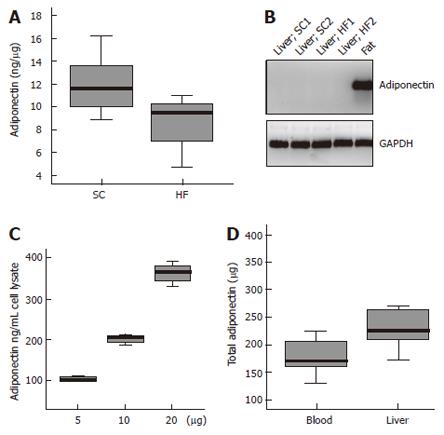
Systemic and liver adiponectin were measured by ELISA. Circulating adiponectin is significantly reduced in fat-fed animals with 4.9 ± 1.1 mg/L adiponectin compared to 6.2 ± 8 mg/L in SC fed rats (P = 0.02) (not shown). Liver adiponectin is also lower in fat rats with 8.5 ± 2.4 ng adiponectin in 1 μg liver tissue when compared to controls with 11.9 ± 1.9 ng adiponectin (P = 0.01) (Figure 2A).
Adiponectin in the liver may be derived from the circulation or originate from liver cells. Adiponectin mRNA was analysed by RT-PCR with total RNA isolated from rat liver of HF and SC animals and rat adipose tissue as a positive control. Adiponectin mRNA was not detected in any RNA isolated from total liver by RT-PCR and 45 cycles of amplification but was easily amplified from adipose tissue RNA (Figure 2B). In addition, adiponectin mRNA was not detected in human liver, in isolated primary human hepatocytes or in HepG2 cells (not shown). Therefore hepatic adiponectin is most likely taken up by liver cells.
To test this hypothesis primary human hepatocytes were incubated with 5, 10 and 20 mg/L recombinant adiponectin for 24 h or PBS as solvent control in serum-free medium. Whereas adiponectin was not detected in the control cells and therefore is below 40 ng/L, adiponectin was found in the cell lysates of hepatocytes incubated with increasing amounts of recombinant protein and was 111 ± 3 μg/L, 200 ± 18 μg/L and 360 ± 43 μg/L, respectively (Figure 2C). This indicates that about 0.02% of the extracellular adiponectin is taken up by hepatocytes and the uptake correlates to the adiponectin concentration in the medium.
Total circulating adiponectin in rats was calculated and the blood volume was estimated as described (Blood volume (mL) = 0.06 × body weight + 0.77) [17]. Total liver adiponectin was also calculated by multiplication of hepatic adiponectin concentration with the corresponding weight of the liver. Adiponectin in the blood was 178 ± 31 μg and in the liver 241 ± 59 μg indicating that total liver adiponectin is higher than circulating adiponectin (P = 0.03) (Figure 2D). However, this is only a rough estimate because the blood volume of the liver was not included in the calculation. Furthermore, adiponectin may be not equally distributed in the whole liver and levels may vary between different biopsies taken from the same organ.
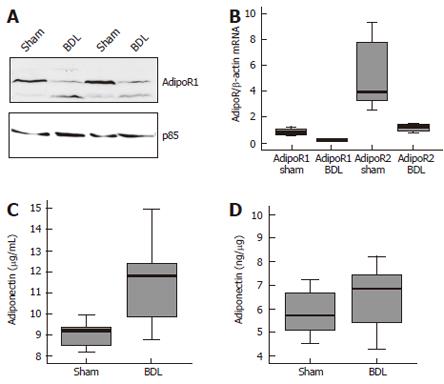
AdipoR1 and AdipoR2 were also determined in a rodent model of liver cirrhosis. AdipoR1 protein was analysed in the liver of control mice and animals after BDL by immunoblot and was found reduced in all BDL animals investigated (Figure 3A). AdipoR1 and AdipoR2 mRNA were determined by real-time RT-PCR in control and BDL mice. Relative abundance of AdipoR1 mRNA was 0.8 ± 0.3 in sham and 0.2 ± 0.1 in BDL mice (P = 0.0004), AdipoR2 mRNA was 5.2 ± 2.6 in controls and 1.2 ± 0.3 in BDL animals (P = 0.007) (Figure 3B). Normalization was performed using β-actin as housekeeping gene which was similarly expressed in both groups (P = 0.14). Systemic adiponectin was significantly higher in the BDL group with 9.0 ± 0.6 mg/L in the control and 11.1 ± 2.0 mg/L in the BDL animals (P = 0.03) (Figure 3C) whereas hepatic adiponectin was similar with 5.8 ± 1.0 mg/g in sham and 6.4 ± 1.5 mg/g in the BDL mice (P = 0.4) (Figure 3D).
To identify mediators that are responsible for the suppression of AdipoR1 in liver cirrhosis, primary hepatocytes and HepG2 cells were incubated for 24 h with either LPS (1 and 10 mg/L), TNF (10 μg/L), supernatants of activated hepatic stellate cells, CCl4 (1 mmol/L and 3 mmol/L) or actinomycin D (10 mg/L). AdipoR1 protein was not found reduced in cells treated with these mediators (not shown).
Although AdipoR2 has been suggested to represent the main adiponectin receptor in the liver we recently demonstrated significant expression of AdipoR1 mRNA and protein in primary human hepatocytes and HepG2 cells[8]. In the current study AdipoR1 mRNA and protein, AdipoR2 mRNA and abundance of circulating and hepatic adiponectin in rodent models of steatotic and cirrhotic liver was investigated.
Rats fed a HF diet develop insulin resistance and fatty liver disease[12]. AdipoR1 mRNA and protein is similar when total liver tissue isolated from rats on a standard diet or HF animals was analyzed. Furthermore, insulin did not alter AdipoR1 mRNA or protein in HepG2 cell. Leptin deficient ob/ob mice are obese and insulin resistant and hepatic AdipoR1 mRNA was found similarly expressed in these animals when compared to controls[18]. These data indicate that reduced insulin sensitivity is not associated with reduced AdipoR1 expression in the liver.
AdipoR2 mRNA is about 25% higher in the fatty liver of HF rats whereas in the ob/ob mice AdipoR2 mRNA is not altered[18]. AdipoR2 mRNA was also investigated in humans and is significantly elevated in fatty liver disease when compared to normal liver[19] in one study whereas a second study could not detect alterations in AdipoR2 mRNA[10,20]. However, protein expression has not been investigated so far but is important to clarify abundance of hepatic AdipoR2 in the metabolic syndrome.
Systemic adiponectin is significantly lower in the HF animals and reflects the human situation where circulating adiponectin has an inverse proportion to the body mass index[2]. In accordance with the lower systemic adiponectin levels, hepatic adiponectin is also reduced in the fat rat. Adiponectin mRNA was not detected in rat liver indicating that hepatic adiponectin may be taken up by the cells. Adiponectin was not found in primary human hepatocytes cultivated in vitro for three days but incubation of these cells with 5, 10 or 20 mg/L recombinant adiponectin for 24 h elevated cellular adiponectin in a concentration dependent way. This may indicate that adiponectin is taken up by hepatocytes either from the circulation or the portal vein.
The common and final state of all chronic liver diseases is liver cirrhosis. A well known model of extrahepatic biliary obstruction is common bile duct ligation in mice[21]. AdipoR1 mRNA and protein and AdipoR2 mRNA were found reduced in the cirrhotic liver of BDL animals. Systemic adiponectin is elevated in animals with liver cirrhosis, a phenomenon also described in a study investigating mice and humans[22]. However, hepatic adiponectin was similar in sham and BDL mice. It was suggested that adiponectin is excreted via the bile and an impaired liver function in cirrhosis may reduce biliary loss of circulating adiponectin explaining elevated systemic levels[22]. However, this hypothesis presumes reduced hepatic adiponectin concentrations when compared to control animals and therefore is not supported by our findings.
Although adiponectin was detected in the liver of mice and rats, adiponectin mRNA could not be amplified in rat liver, human liver or isolated human hepatocytes indicating that adiponectin is not produced by hepatocytes or other cells of the liver tissue. These results are in agreement with several studies[10,20] but contradict the results of other reports[11,22] and there is currently no explanation for these different findings. However, hepatocytes take up significant amounts of adiponectin and this may explain at least in part why adiponectin is detected in the liver.
One limitation of the current study is that only four to eight animals could be analyzed per experiment and the investigations would benefit from a higher number of rodents. Nevertheless low circulating adiponectin in obesity[2] and high levels in liver cirrhosis[22] have also been reported in human studies and are in accordance with our findings.
All in all, our experiments show reduced circulating adiponectin in a rodent model of fatty liver disease and elevated adiponectin and diminished expression of adiponectin receptors in BDL-induced liver cirrhosis in mice.
The expert technical assistance of Kerstin Winkler, Iris Ottinger and Anja Graebe is greatly appreciated.
| 1. | McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Gil-Campos M, Cañete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem. 2002;277:29359-29362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 288] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin. Hepatology. 2004;40:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 696] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [PubMed] |
| 6. | Czaja MJ. Liver injury in the setting of steatosis: crosstalk between adipokine and cytokine. Hepatology. 2004;40:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2340] [Article Influence: 101.7] [Reference Citation Analysis (1)] |
| 8. | Neumeier M, Weigert J, Schäffler A, Weiss T, Kirchner S, Laberer S, Schölmerich J, Buechler C. Regulation of adiponectin receptor 1 in human hepatocytes by agonists of nuclear receptors. Biochem Biophys Res Commun. 2005;334:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 9. | Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341-18347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Vuppalanchi R, Marri S, Kolwankar D, Considine RV, Chalasani N. Is adiponectin involved in the pathogenesis of nonalcoholic steatohepatitis A preliminary human study. J Clin Gastroenterol. 2005;39:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Buettner R, Ottinger I, Schölmerich J, Bollheimer LC. Preserved direct hepatic insulin action in rats with diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2004;286:E828-E833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hellerbrand SC, Tsukamoto H, Brenner DA, Rippe RA. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Weiss TS, Pahernik S, Scheruebl I, Jauch KW, Thasler WE. Cellular damage to human hepatocytes through repeated application of 5-aminolevulinic acid. J Hepatol. 2003;38:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Thasler WE, Weiss TS, Schillhorn K, Stoll PT, Irrgang B, Jauch KW. Charitable State-Controlled Foundation Human Tissue and Cell Research: Ethic and Legal Aspects in the Supply of Surgically Removed Human Tissue For Research in the Academic and Commercial Sector in Germany. Cell Tissue Bank. 2003;4:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Buechler C, Ullrich H, Aslanidis C, Bared SM, Lingenhel A, Ritter M, Schmitz G. Lipoprotein (a) downregulates lysosomal acid lipase and induces interleukin-6 in human blood monocytes. Biochim Biophys Acta. 2003;1642:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72-76. [PubMed] |
| 18. | Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817-30822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 419] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Takamura T, Shimizu A, Matsuzawa N, Honda M, Kaneko S. Adiponectin receptor II in non-alcoholic fatty liver disease. Gut. 2005;e-letter to reference 11. |
| 20. | Oana F, Takeda H, Matsuzawa A, Akahane S, Isaji M, Akahane M. Adiponectin receptor 2 expression in liver and insulin resistance in db/db mice given a beta3-adrenoceptor agonist. Eur J Pharmacol. 2005;518:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Rodríguez-Garay EA. Cholestasis: human disease and experimental animal models. Ann Hepatol. 2003;2:150-158. [PubMed] |
| 22. | Tacke F, Wüstefeld T, Horn R, Luedde T, Srinivas Rao A, Manns MP, Trautwein C, Brabant G. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Schreyer AG E- Editor Ma WH