Published online Sep 14, 2006. doi: 10.3748/wjg.v12.i34.5458
Revised: January 8, 2006
Accepted: January 24, 2006
Published online: September 14, 2006
Infection with H pylori leads to a persistent chronic inflammation of the gastric mucosa, thereby increasing the risk of distal gastric adenocarcinoma. Numerous studies have determined a clear correlation between H pylori infection and the risk of gastric cancer; however, general eradication is not recommended as cancer prophylaxis and time points for treatment remain controversial in different areas of the world. Prevalence rates in Western countries are decreasing, especially in younger people (< 10%); and a decline in distal gastric adenocarcinoma has been observed. Risk groups in Western countries still show considerably higher risk of developing cancer, especially in patients infected with cagA+ strains and in persons harboring genetic polymorphism of the IL-1B promoter (-511T/T) and the corresponding IL-1 receptor antagonist (IL-1RN*2). Thus, general eradication of all infected persons in Western countries not recommended and is limited to risk groups in order to achieve a risk reduction. In contrast, infection rates and cancer prevalence are still high in East Asian countries. A prevention strategy to treat infected persons may avoid the development of gastric cancer to a large extent and with enormous clinical importance. However, studies in China and Japan indicate that prevention of gastric cancer is effective only in those patients that do not display severe histological changes such as atrophy and intestinal metaplasia. Thus, prophylactic strategies to prevent gastric cancer in high risk populations such as China should therefore especially aim at individuals now at younger age when the histological alterations caused by the bacterial infection was still reversible. In countries with a low prevalence of gastric cancer, risk groups carrying cagA+ strains and IL-1 genetic polymorphisms should be identified and treated.
- Citation: Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: Shifting the global burden. World J Gastroenterol 2006; 12(34): 5458-5464
- URL: https://www.wjgnet.com/1007-9327/full/v12/i34/5458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i34.5458
Since the discovery of H pylori in 1983, intensive research has led to the conclusion that infection with this bacterium is the major cause for the development of distal gastric cancer. Infection with the bacterium leads to a chronic inflammation of the gastric epithelium, associated with multifocal gastric atrophy, dysplasia, and malignant transformation in some of the infected patients[1-6]. Controversy remains why only a minority of infected patients develops distal adenocarcinoma, and how geographic differences between Western and Asian countries may contribute to these differences. H pylori infection rates average at about 30% in Western populations; in this part of the world, approximately 0.1%-1% of the patients with H pylori induced gastritis will develop distal gastric cancer[7,8]. Infection rates in Asian countries are higher and range at 60%-88%; distal gastric adenocarcinoma is even more frequent in these countries. Overall, gastric cancer was responsible for almost 650 000 deaths worldwide in the year 2000[9]. Death from gastric cancer is second only to lung cancer in men and thus contributes to approximately 10% of all cancer deaths annually (see: http://www.who.org). Since the diagnosis of early gastric cancer is difficult and in most cases diagnosis is made at a more progressed stage, treatment becomes cost-intensive. Therefore, there is a considerable interest to understand the underlining mechanisms and to find strategies to eradicate H pylori infection which could prevent gastric carcinogenesis. Such statistical calculations have already been anticipated 10 years ago in high risk populations of Japanese immigrants in Hawaii; currently, however, no guidelines exist when and whom to treat[10,11].
In Germany in the year 2000, 21 000 patients suffered from gastric cancer with an equal distribution among males and females[12]. In the US (http://seer.cancer.gov), a similar occurrence has been reported with more than 45 000 deaths per year. In countries such as the USA or Germany, the prevalence of H pylori is decreasing, so that meanwhile less than 10% of the children are infected with this bacterium, underlining that a prophylactic eradication or vaccination in childhood is of little interest in these countries[8,13-15]. Nevertheless, infection rates are high in elderly populations above the age of 50, and this group is particularly suffering from gastric cancer. A reduction of cancer risk may be achieved when patients are cured from the infection. It may thus be important to identify those patients who are at increased risk.
Gastric cancer is the second highest cause of cancer-related deaths in Japan, and the death rate due to gastric adenocarcinoma has actually marginally increased to approximately 50 000 deaths per year in this country[13,14,16]. In China, a similar prevalence of gastric cancer has been reported. H pylori infection rates in these countries are high and range from 60% in the younger population (10-40 years old) to 80%-90% in elderly patients above the age of 50. In Japan, mortality from distal gastric adenocarcinoma is among the highest scores of the world and could be attributed to the very high rates of H pylori infection in this country and the fact that the most common strain of H pylori found in Japan is extremely virulent. A prophy-lactic eradication in people at younger age could save millions of lives as well as enormous costs resulting from ulcer disease, gastrointestinal bleeding and gastric cancer treatment. Therefore, a successful eradication of this pathogen is of crucial socio-economical importance especially in countries such as Japan or China, particularly especially in persons who are at age < 50 years.
Although there is a considerable high rate of H pylori infection in Asian countries such as China, Japan, Thailand and Indonesia, there is a remarkable difference regarding the outcome of gastric cancer within these countries. This observation has also been termed as the “Asian paradox”: Although there is a high infection rate in Thailand and Indonesia, there is only little risk of developing gastric cancer in these countries[14,17]. As discussed below, bacterial virulence factors as well as the individual host immune response may be different among those countries and account for the differential development of the infection outcome. Questions remain whether to treat all infected patients, subgroups of patients, or wait until serious histological alterations have occurred in the various countries mentioned above. The outcome of infection, however, is difficult to predict. Recent studies have elucidated some of the mechanisms of chronic inflammation and thus enable a more precise prediction of the clinical course of infection in different countries.
In recent studies, a new analytical investigation to overcome a potential underestimation of the association of H pylori with gastric cancer was performed in a Western population. Applying various more stringent exclusion criteria to minimize a potential bias from this source increased the odds ratio (95% confidence interval) of non-cardia gastric cancer from 3.7 to 18.3 for any H pylori infection, and from 5.7 to 28.4 for cagA+ H pylori infections in Germany[18-20]. A similar approach has been performed in a study from Sweden, and the results were remarkably consistent with the German study. The observations made in these studies are further supported by a recent cohort study from Japan, in which 36 out of 1246 H pylori infected subjects, but none of 280 uninfected subjects developed gastric cancer during a mean follow-up of 7.8 years[21]. These very stringent epidemiological studies strongly support that the H pylori gastric cancer association may have been strongly underestimated the risks in previous studies, and consequently, underline that infection especially with certain cagA+ H pylori can be considered as a true carcinogenic factor. Determination of cagA status may thus help the physician to identify people who are at increased risk for gastric cancer. Meanwhile, tests have become available as a serological test and a specific antibody staining for histological specimens[16].
Bacterial virulence factors, especially genetic diversity in the cagA region, have been claimed to account for this diverging development. The H pylori cag pathogenicity island (cagPAI) is a 35 to 40 kb genetic element that encodes a type IV secretion system. One of the key factors of this system is the CagA protein; cagA+ strains inject the CagA protein directly into host cells where it undergoes tyrosine phosphorylation by a host-cell kinase, and the phosphoprotein alters the physiology of the affected cells. Huang et al conducted a meta-analysis of the relationship between CagA seropositivity and gastric cancer[22]. Based on 7 studies with 1707 gastric cancer patients and 2124 matched controls the analysis showed that infection with cagA+ H pylori strains increases the risk for gastric cancer 2.87 fold over the risk associated with H pylori infection alone.
Hatatekayama et al recently reported that CagA proteins isolated in East Asia, where gastric cancer is prevalent, have a distinct sequence at the phosphorylation site compared with CagA proteins from Western H pylori strains[23-25]. This CagA diversity may be one important variable in determining the biological activity of CagA and the clinical outcome of infection. Translocated CagA forms a physical complex with the SRC homology 2 domain (SH2)-containing tyrosine phosphatase (SHP-2) in a phosphorylation-dependent manner, and stimulates phosphatase activity. SHP-2 is known to play an important inductive role in mitogenic signal transduction. Deregulation of SHP-2 by CagA may induce abnormal proliferation of gastric epithelial cells. In addition, the CagA protein is polymorphic. Thus, CagA proteins isolated in northern parts of East Asia, where gastric cancer is prevalent, have a distinct sequence at the phosphorylation site and thus contribute to the differential outcome.
cagA+ H pylori strains also are more likely to express the babA product, which mediates adherence to Lewisb antigens on gastric epithelial cells[26]. European studies have determined that bacteria expressing the adherence factor BabA are detected more frequently in patients with gastric cancer or severe histological changes in the mucosa. Comparing several independent studies in different countries, the relative risk for the development of distal gastric adenocarcinoma was increased up to 20-fold. These results support the hypothesis, that the risk of developing severe gastric pathologies is dramatically increased once the correct determination of the cagA status is performed[27-31].
A new concept has now emerged why cagA+/babA+H pylori strains induce higher levels of the proinflamma-tory cytokines IL-1β and IL-8. Adherence of H pylori to epithelial cells via BabA favors a more tight adhesion to the epithelial cell and thus promotes bacterial colonisation. Injection of CagA into epithelial cells is associated with IL-8 secretion, which in turn, acts as a local chemoattractant for polymorphic mononuclear cells (PMN)[29]. These PMNs are considered to be of critical importance for the breakdown of the local epithelial barrier and may lead to a further infiltration of the bacteria into the submucosa. Thus, several studies outline the view that bacterial virulence and adherence factors contribute to the development of severe diseases in the stomach. Infection with certain H pylori strain types is therefore more dangerous than smoking a pack of cigarettes per day, leading to a 21-fold increased relative risk for the development of lung cancer.
Infection with so called type 1 strains harboring cagA/vacAs1 and babA is associated with a dramatically increased risk to develop distal gastric adenocarcinoma. Infection with specific strain types thus is the major determinant of the further sequence of events.
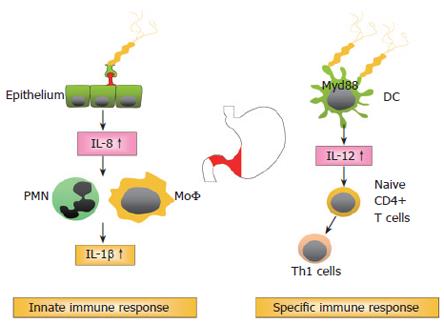
In addition to bacterial factors, but less important in terms of relative risk increment, are host factors that seem to influence the inflammatory response and the development of a more severe pathology. H pylori induced inflammation is implicated in the development of mucosal damage and is characterised by strong granulocytic and lymphocytic infiltration. The T helper cell response towards H pylori is generally considered to be of the Th1 phenotype, leading to a cell mediated immune response[32-35] (Figure 1). There is increasing evidence that the H pylori induced Th1 response contributes to cancer development. Downregulation of the Th1 response in mice by concurrent enteric helminth infection or p53 mutation was shown to protect against the development of atrophy, intestinal metaplasia, and invasive gastric carcinoma[35-37]. Important cytokines characterising Th1 mediated immune responses are interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), all being upregulated during chronic H pylori infection[38-40]. IL-10, which is also highly expressed in the H pylori infected stomach, is one of the most important regulatory cytokines, inhibiting cell mediated immune responses[38,41,42].
Genes encoding cytokines and related molecules harbour polymorphic regions, which are considered to alter gene transcription and thereby influence inflammatory processes in response to infectious diseases. Polymorphisms in the human IL-10, IL-1B, TNF-α, IFN-γ, and IL-1 receptor antagonist (IL-1RN) genes have been reported to influence cytokine expression[43-47]. In the promoter region of the IL-1B gene, IL-1B–511T, which is in complete linkage disequilibrium with IL-1B–31C, was previously associated with slightly, but not significantly, increased IL-1β secretion from stimulated PBMC. The IL-1RN gene has a penta-allelic 86 bp variable number of tandem repeat region (VNTR) in intron 2, of which allele 2 (IL-1RN*2) was previously associated with enhanced IL-1β secretion[46] and the development of gastric cancer[44]. Presence of certain genetic polymorphisms of the host, in contrast to the bacterial factors, increase the relative risk for distal gastric adenocarcinoma by 1.5-4 fold. In a German population[48], the homozygous genotype IL-1RN*2/2 of the IL-RN gene was strongly associated with early-stage gastric cancer (P < 0.0001), whereas further associations with the IL-1 gene cluster were not observed. A Korean group[49] determined that patients with intestinal-type gastric cancer showed a higher frequency of IL-1B-31T homozygotes [odds ratio (OR) = 2.2; 95% confidence interval (CI) = 1.1-4.3] compared with controls. Risk was also significantly increased in these patients for IL-1B-31T homozygotes compared with patients with diffuse-type gastric cancer (OR = 3.4; 95% CI = 1.5-7.7). In a Chinese study, it was reported that the relative risks associated with the IL-1B variant genotypes were 1.64 (95% CI, 1.01-2.66) for -31TT and 1.52 (95% CI, 0.91-2.54) for -511CC, respectively, compared with their wild-type homozygotes[50]. The risks were significantly more evident in individuals with H pylori infection (adjusted OR = 2.14; 95% CI, 1.13-4.06 for -31TT; adjusted OR = 2.00; 95% CI, 1.02-3.89 for -511CC), which was consistent with the biological effects of IL-1β.
Chen et al[51] identified that the carriage of IL-1RN*2 polymorphism, male gender, old age and H pylori infection independently increased the risk of gastric cancer, with odds ratios of 3.3 (95% CI, 1.4-7.7), 2.1 (95% CI, 1.2-3.8), 5.3 (95% CI, 3.1-9.0) and 2.2 (95% CI, 1.3-3.8), respectively. H pylori-infected individuals who were carriers of IL-1RN*2 showed increased risks for both intestinal and diffuse types of gastric cancer, with odds ratios of 11.0 and 8.7, respectively. Thus, IL-1RN*2 was interpreted to be an independent factor governing the development of gastric cancer in Asian individuals.
In areas of high and low prevalence of gastric cancer in China[52], the IL-1B -511T/T genotype frequency was significantly higher among patients with gastric cancer (25.0%) than control subjects (12.5%) in a region with low GC prevalence. While H pylori infection alone had only a modest effect on the risk of gastric cancer development (OR = 5.0, 95% CI 1.5-16.3), combined with the IL-1B -511T/T genotype the risk was markedly elevated (OR = 17.1, 95% CI 3.8-76.4). The study underlines the synergy between H pylori infection and the host immune response to induce gastric cancer.
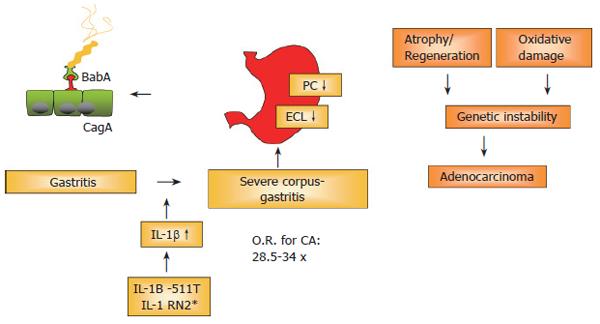
A recent German study confirmed this synergistic effect also in regard to the induction of premalignant changes. In a large group of H pylori infected patients[29], carriers of the proinflammatory IL-1B -511T/-31C and IL-1RN*2 alleles had an increased risk for the development of atrophic gastritis (AG), intestinal metaplasia (IM), and severe inflammation, ORs of 1.7 (95% CI, 0.8-3.4) to 4.4 (95% CI, 1.5-12.9). The highest prevalence of severe gastric abnormalities was found in patients with both host and bacterial high-risk genotypes (cagA+/vacAs1+/IL-1B -511T/IL-1RN*2), with ORs of 24.8 (95% CI, 5.2-117.3) for severe lymphocytic infiltration, 9.5 (95% CI, 2.8-32.1) for severe granulocytic infiltration, 6.0 (95% CI, 2.4-15.5) for IM, and 2.4 (95% CI, 0.93-6.2) for AG. These dramatically elevated values underline the importance of the infection for gastric cancer development. It may thus be concluded from the above studies, that combined bacterial/host genotyping thus may provide a clinical tool to identify patients at high risk of developing gastric cancer. This current concept of gastric carcinogenesis is illustrated in Figure 2.
There is overwhelming evidence that H pylori infection, especially with cagA+ strains, leads to a strong granulocytic and lymphocytic infiltration; a subgroup of infected patients will develop gastric cancer, especially in high-risk countries such as Japan, China and East-European countries. Reasons for this progression include genetic polymorphisms, e.g. IL-1B/IL-1RN polymorphisms associated with high levels of IL-1β. IL-1β is a strong antisecretory cytokine. These genetic polymorphisms lead to a heightened cytokine release, which in turn decreases acid secretion. Subsequently, the association of hypochlorhydric conditions with the persistent inflammation in the gastric corpus is considered to be a true risk factor (RR: 34.5-fold!) for the development of gastric cancer. Studies should therefore aim not only at preventing gastric cancer, but also at preventing the development of severe corpus gastritis, leading to potentially irreversible atrophy and hypochlorhydria.
Data from a Chinese trial[53] investigating H pylori eradication in gastric cancer showed that the incidence of gastric adenocarcinoma development was similar in patients undergoing eradication therapy compared with placebo over a 7.5-year period. Interestingly, in a subgroup of H pylori-positive patients without atrophy, intestinal metaplasia or dysplasia (pre-cancerous lesions), eradication therapy significantly decreased the development of gastric adenocarcinoma to a frequency of zero. The data are in accordance with a recent study in Japan[4]: in this prospective study, no carcinomas were observed in the H pylori negative group. This study supports the view that a prospective eradication study should be performed in young adults in which histological alterations have not proceeded too far and may be reversible.
Zhou et al[54] investigated histopathological changes in H pylori eradicated subjects in China over a period of more than 5 years. The authors report in a group of 552 H pylori-positive subjects that, the severity and activity of inflammation in both the antrum and body were markedly reduced after H pylori eradication. Within the five years after eradication of H pylori, intestinal metaplasia in the antrum regressed or showed no progression, while the proportion of intestinal metaplasia in the H pylori-positive group increased significantly. After H pylori eradication, the atrophy in both the antrum and body had no significant regression. After eight years of observation, the authors report a significantly higher incidence of gastric cancer in the H pylori infected group (5/1530) than in the H pylori-negative (1/1230) group in the Yantai area of China, underlining the need for eradication in early stages.
A recently published study[55] reports that subjects seropositive for either H pylori or CagA who had low pepsinogen (PG) I levels had the highest OR (9.21 95% CI 4.95-17.13) for noncardia cancer, compared with subjects with neither factor. The results suggest that individuals with both H pylori or CagA seropositivity and a low PG Ilevel or low PGI/II ratio are highly susceptible to development of noncardia gastric cancer. Such tests can easily be performed in clinical routine. Watabe et al[56] have confirmed that H pylori infection and gastric atrophy are both risk factors for gastric cancer in a study group with a total of 9293 participants. The annual incidence of gastric cancer was 0.04%-0.60%. As expected, the highest relative risk for gastric cancer was found in H pylori infected subjects with gastric atrophy and high serum pepsinogen levels [OR = 8.2 (3.2-21.5)].
Concerns exist whether eradication of H pylori might lead to the development of esophageal cancer. To clarify the role of H pylori infection in these tumors with divergent incidence trends, Chow et al[57] analyzed serum IgG antibodies to H pylori and to a recombinant fragment of CagA by antigen-specific ELISA among 129 patients newly diagnosed with esophageal/gastric cardia adenocarcinoma, 67 patients with noncardia gastric adenocarcinoma, and 224 population controls. Infection with cagA+ strains was not significantly related to risk for noncardia gastric cancers (OR = 1.4; CI = 0.7-2.8) but was significantly associated with a reduced risk for esophageal/cardia cancers (OR = 0.4; CI = 0.2-0.8). The study thus contradicts previous reports and may be biased by the selection of patients in a rather small group. While Wu et al[58] found no significant association, Ye et al[59] determined the opposite: H pylori infection may protect from esophageal cancer (OR = 0.3). Thus, at this point, several works indicate that CagA has a protective effect on the development of esophageal cancer, however, no general conclusions can be made.
However, this association does not justify a general refusion of H pylori eradication because of two reasons. First, esophageal adenocarcinoma is far less common than gastric cancer. Second, the risk of developing adenocarcinoma of the esophagus is lowered by a factor 2-2.5 in the presence of cagA-positive H pylori infection whereas the risk for distal gastric cancer associated with cagA-positive strains is much higher (5-28x).
H pylori is a clear cut carcinogen. Infection with certain strain types in the presence of genetic polymorphisms leading to a heightened inflammatory response is associated with a dramatically increased relative risk to develop gastric cancer. Such developments can be foreseen by evaluating gastric biopsies, but also by determining serum pepsinogen levels as a marker of gastric atrophy. Endoscopy of the upper gastrointestinal tract (GI) should be performed before eradication to determine the status of gastric inflammation. Eradication of H pylori as prophylaxis of gastric adenocarcinoma is effective in early stages in which no severe histological changes have occurred. In countries with a high prevalence of infection and high cancer risks such as Japan or China, a general prophylactic eradication strategy seems to be beneficial especially in younger patients (< 50 years); nevertheless, the outcome of such long term prospective prophylactic studies needs to be evaluated. In Western countries with low prevalence rates and low cancer rates, a test-and-treat strategy is not cost-effective to prevent cancer; however, identification and treatment of risk groups seems rationale. Risk groups can be identified by evaluation of family history, presence of histological alterations in the gastric corpus, or by determining infection with cagA+ strains as well as determining the status of genetic polymorphisms in the IL-1RN*2 gene.
| 1. | Blaser MJ. The biology of cag in the Helicobacter pylori-human interaction. Gastroenterology. 2005;128:1512-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [PubMed] |
| 3. | Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3247] [Article Influence: 129.9] [Reference Citation Analysis (1)] |
| 5. | Sipponen P. Gastric cancer: pathogenesis, risks, and prevention. J Gastroenterol. 2002;37 Suppl 13:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Sipponen P, Marshall BJ. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am. 2000;29:579-592, v-vi. |
| 7. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1934] [Article Influence: 80.6] [Reference Citation Analysis (3)] |
| 8. | Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 765] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 472] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1240] [Article Influence: 35.4] [Reference Citation Analysis (2)] |
| 11. | Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Sendler A, Böttcher K, Etter M, Siewert JR. [Gastric carcinoma]. Internist (Berl). 2000;41:817-818, 821-826, 828-830. [PubMed] |
| 13. | Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Go MF. Diagnosis and Treatment of Helicobacter pylori. Curr Treat Options Gastroenterol. 2005;8:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Matsukura N, Yamada S, Kato S, Tomtitchong P, Tajiri T, Miki M, Matsuhisa T, Yamada N. Genetic differences in interleukin-1 betapolymorphisms among four Asian populations: an analysis of the Asian paradox between H. pylori infection and gastric cancer incidence. J Exp Clin Cancer Res. 2003;22:47-55. [PubMed] |
| 18. | Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer. Am J Epidemiol. 2004;159:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Brenner H, Arndt V, Stürmer T, Stegmaier C, Ziegler H, Dhom G. Individual and joint contribution of family history and Helicobacter pylori infection to the risk of gastric carcinoma. Cancer. 2000;88:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Brenner H, Bode G, Boeing H. Helicobacter pylori infection among offspring of patients with stomach cancer. Gastroenterology. 2000;118:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Azuma T, Yamakawa A, Yamazaki S, Ohtani M, Ito Y, Muramatsu A, Suto H, Yamazaki Y, Keida Y, Higashi H. Distinct diversity of the cag pathogenicity island among Helicobacter pylori strains in Japan. J Clin Microbiol. 2004;42:2508-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M. Association between diversity in the Src homology 2 domain--containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004;189:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 592] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 26. | Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikström S, Sjöström R, Lindén S, Bäckström A. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 304] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778-12783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 436] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Prinz C, Schöniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, Classen M, Rösch T, Schepp W, Gerhard M. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61:1903-1909. [PubMed] |
| 29. | Rad R, Gerhard M, Lang R, Schöniger M, Rösch T, Schepp W, Becker I, Wagner H, Prinz C. The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune response. J Immunol. 2002;168:3033-3041. [PubMed] |
| 30. | Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, Gerhard M, Prinz C. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Rad R, Prinz C, Neu B, Neuhofer M, Zeitner M, Voland P, Becker I, Schepp W, Gerhard M. Synergistic effect of Helicobacter pylori virulence factors and interleukin-1 polymorphisms for the development of severe histological changes in the gastric mucosa. J Infect Dis. 2003;188:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 430] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 33. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 404] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Wang J, Brooks EG, Bamford KB, Denning TL, Pappo J, Ernst PB. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J Immunol. 2001;167:926-934. [PubMed] |
| 36. | Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res. 2002;62:696-702. [PubMed] |
| 37. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004;12:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Bodger K, Bromelow K, Wyatt JI, Heatley RV. Interleukin 10 in Helicobacter pylori associated gastritis: immunohistochemical localisation and in vitro effects on cytokine secretion. J Clin Pathol. 2001;54:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997;40:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 680] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 44. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, Pérez-Pérez GI. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515-G520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 47. | Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Glas J, Török HP, Schneider A, Brünnler G, Kopp R, Albert ED, Stolte M, Folwaczny C. Allele 2 of the interleukin-1 receptor antagonist gene is associated with early gastric cancer. J Clin Oncol. 2004;22:4746-4752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Chang YW, Jang JY, Kim NH, Lee JW, Lee HJ, Jung WW, Dong SH, Kim HJ, Kim BH, Lee JI. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1beta cytokine in Korean patients with gastric cancer. Int J Cancer. 2005;114:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Yang J, Hu Z, Xu Y, Shen J, Niu J, Hu X, Guo J, Wei Q, Wang X, Shen H. Interleukin-1B gene promoter variants are associated with an increased risk of gastric cancer in a Chinese population. Cancer Lett. 2004;215:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Chen A, Li CN, Hsu PI, Lai KH, Tseng HH, Hsu PN, Lo GH, Lo CC, Lin CK, Hwang IR. Risks of interleukin-1 genetic polymorphisms and Helicobacter pylori infection in the development of gastric cancer. Aliment Pharmacol Ther. 2004;20:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M, Sung JJ. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1051] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 54. | Zhou LY, Shen ZY, Lin SR, Jin Z, Ding SG, Huang XB, Xia ZW, Liu JJ, Guo HL, William C. [Changes of gastric mucosa histopathology after Helicobacter pylori eradication]. Zhonghua Neike Zazhi. 2003;42:162-164. [PubMed] |
| 55. | Nomura AM, Kolonel LN, Miki K, Stemmermann GN, Wilkens LR, Goodman MT, Perez-Perez GI, Blaser MJ. Helicobacter pylori, pepsinogen, and gastric adenocarcinoma in Hawaii. J Infect Dis. 2005;191:2075-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 57. | Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, Perez-Perez GI, Schoenberg JB, Stanford JL, Rotterdam H. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588-590. [PubMed] |
| 58. | Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, Nyrén O. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Rampone B E- Editor Liu WF