Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5229
Revised: May 15, 2006
Accepted: May 22, 2006
Published online: August 28, 2006
Experimental evidence indicates that chronic mechanical sub-occlusion of the intestine may damage the enteric nervous system (ENS), although data in humans are lacking. We here describe the first case of enteric degenerative neuropathy related to a congenital obstruction of the gut. A 3-year and 9-mo old girl began to complain of vomiting, abdominal distension, constipation with air-fluid levels at plane abdominal radiology.
Her subsequent medical history was characterized by 3 operations: the first showed dilated duodeno-jejunal loops in the absence of occlusive lesions; the second (2 years later) was performed to obtain full-thickness biopsies of the dilated intestinal loops and revealed hyperganglionosis at histopathology; the third (9 years after the hyperganglionosis was identified) disclosed a Ladd’s band which was removed and the associated gut malrotation was corrected. Repeated intraoperative full-thickness biopsies showed enteric degenerative neuropathy along with reduced interstitial cells of Cajal network in dilated loops above the obstruction and a normal neuromuscular layer below the Ladd’s band.
One year after the latest surgery the patient tolerated oral feeding and did well, suggesting that congenital (partial) mechanical obstruction of the small bowel in humans can evoke progressive adaptive changes of the ENS which are similar to those found in animal models of intestinal mechanical occlusion. Such ENS changes mimic neuronal abnormalities observed in intestinal pseudo-obstruction.
- Citation: Nardo GD, Stanghellini V, Cucchiara S, Barbara G, Pasquinelli G, Santini D, Felicani C, Grazi G, Pinna AD, Cogliandro R, Cremon C, Gori A, Corinaldesi R, Sanders KM, Giorgio RD. Enteric neuropathology of congenital intestinal obstruction: A case report. World J Gastroenterol 2006; 12(32): 5229-5233
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5229.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5229
The enteric nervous system (ENS), the third component of the autonomic nervous system, plays a crucial role in the control of gastrointestinal functions, including motility, secretion, absorption, blood flow, mucosal growth and aspects of the local immune system[1]. Hence, any condition altering the integrity of the ENS is responsible for a wide spectrum of disorders affecting the gastrointestinal tract. Abnormalities of the ENS (also referred to as enteric neuropathies) may be secondary to a variety of inflammatory, infectious, metabolic and neurological diseases, or they can be labelled idiopathic when no causes can be found[2-6]. The most common clinical manifestation related to an underlying enteric neuropathy (either idiopathic or secondary) is a severe functional impairment of gastrointestinal motility as that identifiable in patients with chronic intestinal pseudo-obstruction (CIPO)[4,7,8]. The diagnosis of CIPO is based on the exclusion of any mechanical lesion occluding the gut lumen[4,7,8]. On the other hand, in animals chronic incomplete mechanical occlusion of the intestine may induce ENS damage[5]. The effects of chronic obstruction on the human ENS are still unknown.
Herein we describe the case of a girl in whom an initial diagnostic work-up suggested a diagnosis of CIPO with an underlying neuropathy characterized by hyperganglionosis of the ENS, whereas a laparotomy about 10 years later showed a chronic congenital obstruction of the small bowel due to a Ladd’s band. Full-thickness gut biopsies, taken during this last laparotomy, showed an enteric degenerative neuropathy in the bowel loops located above the obstruction. Because the correct pathology remained undiagnosed for a long time, this case can be considered a human model of mechanical partial obstruction of the gut. Specifically the case indicates that a long-standing (“chronic”) intestinal sub-occlusion can evoke progressive changes of the ENS similar to those found in animal models, and that the observed ENS changes mimic abnormalities often found in CIPO related to idiopathic neuropathies.
G. S. was a full term baby girl weighing 2040 g at delivery whose clinical history was unremarkable until 3 years and 9 mo of age when she began to complain of post-prandial vomiting (often bilious) and constipation. Clinical examination revealed abdominal distension, dehydration and decreased bowel sounds. Systemic and neurologic diseases, as well as infections and malabsorption syndromes were excluded by physical examination and blood tests. X-ray of the small bowel showed mild gaseous distension of the proximal small bowel loops with air-fluid levels although a CT scan failed to reveal any mechanical obstruction. Due to the severity of the clinical picture and the marked small bowel distension, the patient underwent laparoscopy showing duodeno-jejunal dilatation with no obvious mechanical cause. The patient was discharged with a diagnosis of intestinal pseudo-obstruction and treated with prokinetics and a low-fiber diet.
Subsequently, she had recurrent episodes of vomiting, abdominal pain, early satiety and fullness. Because of the persistence of severe dyspeptic symptoms, at 5 years and 7 mo of age the patient was referred to a tertiary center for pediatric gastroenterology where, based on the clinical history and previous examinations, a decision to undertake an explorative laparotomy was reached. The intra-operative evaluation showed a marked degree of duodeno-jejunal dilatation but no mechanical obstruction.
Analysis of full-thickness biopsies taken from the dilated jejunal loop revealed enlarged myenteric and submucosal neurons whose number was increased as compared to sex-age-matched normal controls[9]. A further important feature was the increased density of the nerve fibers in the lamina propria and submucosa. Based on the histopathology suggesting a case of hyperganglionosis, the diagnosis of CIPO was confirmed and the patient received several courses of prokinetics, metronidazole and high-caloric liquid diet supplementation.
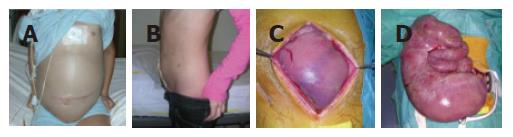
During a subsequent recovery the patient underwent an upper gastro-intestinal manometry which showed a pattern indicative of neurogenic-type CIPO. Due to the progressive worsening of her clinical status, at 14 years and 2 mo of age, the patient was referred to our center in Bologna. Physical examination showed a highly distended (Figure 1A) and painful abdomen, with absence of peristaltic sounds. An X-ray examination showed multiple air-fluid levels in the upper abdomen with a striking elevation of the diaphragm. In order to achieve bowel decompression, she underwent surgery. At laparotomy a Ladd’s band was detected and removed with correction of the associated malrotation. Bowel loops with marked dilatation (Figures 1C and 1D) were resected and a protective ileostomy was created. Full-thickness biopsies were taken from the loops located proximally (dilated segments) and distally (macroscopically normal) to the Ladd’s band.
Following surgery, the clinical course was uneventful with immediate deflation of the abdomen. One year after the last laparotomy the patient was healthy and tolerated oral feeding, her height and weight were markedly increased, and her quality of life was good (Figure 1B).
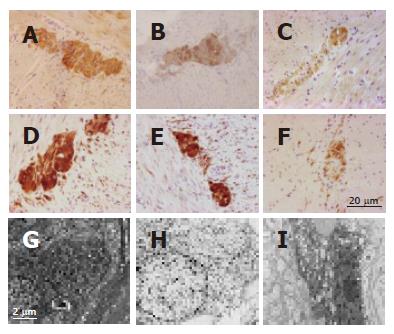
Full-thickness tissue specimens were processed for immunohistochemistry according to standard protocols commonly applied in our laboratory. Compared to controls (jejunal specimens collected from patients operated on for intestinal bleeding due to angiodysplasia; n = 4, 2 females, age range: 6-16 years) (Figure 2A) and the non-dilated segment (Figure 2B), the immunohistochemical evaluation of biopsies taken from the dilated loop showed evidence of intrinsic neuropathy of the gastrointestinal tract characterized by severe myenteric and submucosal neuron depletion (Figure 2C), as identified by the reduced number of neural elements labeled by the general marker neuron specific enolase (NSE) (purchased from DakoCytomation, Milan, Italy). Furthermore, analysis of several transmitters/neuromodulators of the ENS demonstrated a marked decrease of substance P, vasoactive intestinal polypeptide, calcitonin gene-related peptide (all these antibodies were kindly donated by Dr. C. Sternini and H. E. Wong, Center for Ulcer Research and Education/Digestive Diseases Center, UCLA School of Medicine, Los Angeles, CA) and nitric oxide synthase (purchased from BD Biosciences, San Jose, CA) in the myenteric and submucosal neurons and related processes of the dilated segment compared to the non-dilated segment and controls. In order to explore the possible abnormalities of neuronal cell survival, we used a specific antibody against the product of B-cell lymphoma-2 (BCL-2) (DakoCytomation, Milan, Italy), a gene encoding a protein involved in cellular pathways of neuronal apoptosis[10,11]. Compared to the controls (Figure 2D) and the non-dilated segment (Figure 2E), BCL-2 immunoreactivity of the dilated segment was markedly reduced in myenteric (Figure 2F) and submucosal neurons and nerve processes. The interstitial cells of Cajal (ICC), visualized by an antibody against c-Kit (DakoCytomation, Milan, Italy), were markedly decreased in the dilated segment compared to the non-dilated segment and controls. Compared to the controls, the anti-α-smooth muscle actin antibody (DakoCytomation, Milan, Italy) showed an apparently normal muscular layer both in dilated and in non-dilated segments.
Full-thickness tissue specimens were also processed for electron microscopy according to standard protocols commonly applied in our laboratory.
Compared to the controls (Figure 2G) and the non-dilated segment (Figure 2H), electron microscopy analysis of myenteric (Figure 2I) and submucosal neurons of the dilated loop showed degenerative features characterized by nuclear chromatin clumping, shrinkage of the cell body and cytoplasmic vacuoles mostly deriving from the enlargement and matrix clearing of mitochondria.
The present case illustrates the occurrence of gut failure as a result of longstanding mechanical small bowel obstruction due to a Ladd’s band, which has been unrecognized for more than 10 years despite extensive radiological investigation and 3 explorative laparotomies. A Ladd’s band, which arises from the posterior abdominal peritoneum and extends from the liver to the colon (passing anteriorly to the duodenum), is responsible for gut malrotation[12]. Symptomatic patients usually present either acutely with bowel obstruction and intestinal ischemia, or chronically with vague abdominal pain. Chronic symptoms can often make diagnosis difficult, as was the case in our patient. Although Ladd’s band and associated gut malrotation are more common in the first 2 wk of life[13,14], our case provides evidence that patients having this developmental abnormality may be found in childhood.
The clinical and laboratory features of this case have expanded our knowledge on the ENS neuropathology. First, chronic intestinal partial obstruction occurring as a result of a long-standing mechanical cause (Ladd’s band) evokes changes similar to those found in animal models; second, enteric neuropathologic changes observed in the advanced stage of this case could not be differentiated from the neurodegenerative findings often detected in cases of CIPO.
In the current case, early tissue analysis of dilated bowel segments (i.e., from the region which was subsequently identified as proximal to the Ladd’s band) showed hyperganglionosis of the ENS. These neuronal changes, which were reported when the patient was 5 years old, may be considered the result of adaptive phenomena to a long-standing mechanical stimulus, such as obstruction limiting bowel propulsion. Several experimental models have been instrumental in supporting the concept that adaptive changes to the ENS occur proximal to a partially obstructed gut[5,15-21]. These studies showed increased density and size in both myenteric[16,18,19,21] and submucosal neurons[19-21], along with neurochemical[18,20] and cytoskeletal abnormalities of myenteric neurons[21]. Recently, Galvez et al[20] induced surgical stenosis (about 20% of the lumen) of rat sigmoid colon and reported changes in ENS structure after 6-12 wk. The molecular mechanisms involved in enteric neuroplasticity secondary to a partially obstructed bowel remain to be elucidated. Further, enteric neuronal changes, ICC (the pace-maker cells of the gut, which act in concert with neurons in governing gut motility) have been found to be reduced in experimental mechanical sub-occlusion of the small bowel as indicated by a decrease in c-Kit immunoreactivity and loss of functions attributed to ICC[17,18]. In contrast, other data do not support the existence of ICC abnormalities (according to c-Kit immunostaining) in dilated gut segments of patients with Crohn’s disease, a well-known cause of intestinal mechanical sub-occlusion[22]. The possible explanations for the discrepancy between this and other studies remain to be defined.
About nine years after the initial observation of hyperganglionosis, the evaluation of new full-thickness biopsies from the dilated bowel above the stenosis showed evidence of a significant loss of both enteric neurons and ICC, which may explain the marked deterioration of the digestive function observed in our patient. Enteric neuron depletion is supported by the evidence of neurodegenerative changes detected by electron microscopy along with the reduced expression of the protein encoded by BCL-2, a gene related to one of the intracellular pathways involved in the inhibition of programmed cell death[2,3,10,11]. These findings indicate that neuronal cell loss may be due to apoptosis triggered by the persistent mechanical sub-occlusion of the gut. The neurodegenerative abnormalities observed in this phase of the clinical history of the patient may resemble ENS changes described both in humans[23] and in an experimental model of small bowel atresia[24]. In human intestinal atresia, Masumoto et al[23] have shown hypoplasia of myenteric ganglia and marked reduction of both intramuscular nerve fibers and ICC in the dilated bowel segments above atresia. Similarly, in a chick embryo model of intestinal atresia, Schoenberg and Kluth[24] have found an almost complete loss of both ganglionated plexuses in proximal dilated loops.
Taken together, enteric hyperganglionosis followed by degenerative changes can be considered the result of a bi-phasic adaptive process of the ENS in response to a persistent mechanical obstruction of the gut.
A further consideration which can be drawn from the present case concerns the accuracy of ENS pathology in patients with intestinal sub-occlusion. This case report clearly indicates that the enteric neuropathologic changes observed in different stages of a mechanical obstruction could not be distinguished from the neurodegenerative findings often detected in cases of CIPO. Neuropathology of CIPO includes a wide spectrum of ENS changes ranging from hyperganglionosis up to marked reduction of intramural (especially myenteric) neurons associated with swollen cell bodies, variable neurochemical abnormalities and fragmentation and loss of axons sometimes accompanied with proliferation of glial cells[2-5]. If the mechanical obstruction remains unrecognized, as it did in our case, the ENS abnormalities mimic those identifiable in CIPO associated with an underlying neuropathy. Therefore, our case should be considered as a reminder that ENS changes observed in patients with suspected CIPO may be secondary in nature and should not necessarily be interpreted as definitive evidence for a primary neuropathy.
In conclusion, congenital (partial) mechanical obstruction of the upper small bowel leads to progressive adaptive/neuroplastic changes of the ENS similar to those described in experimental models of intestinal occlusion. Interestingly, such ENS abnormalities mimic those often observed in cases of CIPO.
The authors thank Dr. C Sternini and Mrs. HE Wong (Center for Ulcer Research and Education/Digestive Diseases Center, UCLA School of Medicine, Los Angeles, CA) for the generous gift of the following antibodies: rabbit polyclonal anti- substance P/tachykinin (SP/TK8701), mouse monoclonal anti-vasoactive intestinal polypeptide (VIP55), rabbit polyclonal anti-vasoactive intestinal polypeptide (VIP7913), rabbit polyclonal anti-calcitonin gene-related peptide (CGRP 2A-A) and mouse monoclonal anti-calcitonin gene-related peptide (CGRP4901) generated in their laboratories.
| 1. | Furness JB. The enteric nervous system. Oxford: Blackwell Publishing 2005; . |
| 2. | De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | De Giorgio R, Guerrini S, Barbara G, Cremon C, Stanghellini V, Corinaldesi R. New insights into human enteric neuropathies. Neurogastroenterol Motil. 2004;16 Suppl 1:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Di Lorenzo C. Pseudo-obstruction: current approaches. Gastroenterology. 1999;116:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 5. | Kapur RP. Neuropathology of paediatric chronic intestinal pseudo-obstruction and related animal models. J Pathol. 2001;194:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | De Giorgio R, Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil. 2004;16:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Stanghellini V, Corinaldesi R, Barbara L. Pseudo-obstruction syndromes. Baillieres Clin Gastroenterol. 1988;2:225-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Stanghellini V, Cogliandro RF, De Giorgio R, Barbara G, Morselli-Labate AM, Cogliandro L, Corinaldesi R. Natural history of chronic idiopathic intestinal pseudo-obstruction in adults: a single center study. Clin Gastroenterol Hepatol. 2005;3:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Smith VV. Intestinal neuronal density in childhood: a baseline for the objective assessment of hypo- and hyperganglionosis. Pediatr Pathol. 1993;13:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | De Giorgio R, Barbara G, Stanghellini V, F . De Ponti, S. Guerrini, L. Cogliandro, C. Ceccarelli, B. Salvioli, C. Adamo, R. Cogliandro, M. Tonini, R. Corinaldesi. Reduced bcl-2 expression in the enteric nervous system (ENS) as a marker for neural degeneration in patients with gastrointestinal motor disorders (GIMD). Gastroenterology. 2000;118:A4821. [DOI] [Full Text] |
| 11. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2723] [Article Influence: 75.6] [Reference Citation Analysis (7)] |
| 12. | Ladd WE. Congenital obstruction of the small intestine. JAMA. 1933;101:1453-1458. [DOI] [Full Text] |
| 13. | Gamblin TC, Stephens RE Jr, Johnson RK, Rothwell M. Adult malrotation: a case report and review of the literature. Curr Surg. 2003;60:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | von Flüe M, Herzog U, Ackermann C, Tondelli P, Harder F. Acute and chronic presentation of intestinal nonrotation in adults. Dis Colon Rectum. 1994;37:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Barbosa AJ, Tafuri WL. Ganglion cell number in hypertrophic colon above experimental stenosis. Braz J Med Biol Res. 1983;16:165-169. [PubMed] |
| 16. | Brehmer A, Göbel D, Frieser M, Graf M, Radespiel-Tröger M, Neuhuber W. Experimental hypertrophy of myenteric neurones in the pig: a morphometric study. Neurogastroenterol Motil. 2000;12:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Ekblad E, Sjuve R, Arner A, Sundler F. Enteric neuronal plasticity and a reduced number of interstitial cells of Cajal in hypertrophic rat ileum. Gut. 1998;42:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Gabella G. Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J Neurocytol. 1984;13:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Gálvez Y, Skába R, Vajtrová R, Frantlová A, Herget J. Evidence of secondary neuronal intestinal dysplasia in a rat model of chronic intestinal obstruction. J Invest Surg. 2004;17:31-39. [PubMed] |
| 21. | Jew JY, Williams TH, Gabella G, Zhang MQ. The intestine as a model for neuronal plasticity. Arch Histol Cytol. 1989;52 Suppl:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Streutker CJ, Huizinga JD, Campbell F, Ho J, Riddell RH. Loss of CD117 (c-kit)- and CD34-positive ICC and associated CD34-positive fibroblasts defines a subpopulation of chronic intestinal pseudo-obstruction. Am J Surg Pathol. 2003;27:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Masumoto K, Suita S, Nada O, Taguchi T, Guo R. Abnormalities of enteric neurons, intestinal pacemaker cells, and smooth muscle in human intestinal atresia. J Pediatr Surg. 1999;34:1463-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Schoenberg RA, Kluth D. Experimental small bowel obstruction in chick embryos: Effects on the developing enteric nervous system. J Pediatr Surg. 2002;37:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Bai SH