INTRODUCTION
Liver metastases can occur in 25%-35% of patients with colorectal carcinoma[1], and surgical resection has become accepted as a reasonable treatment for patients with liver metastases. However, because of their propensity to spread along epithelial surfaces, liver metastases of colorectal carcinoma sometimes invade the Glisson’s triad[2]. Therefore, in contrast to hepatocellular carcinoma (HCC), bile duct invasions of liver metastases from colorectal carcinoma are seen in about 10%-12% of resected liver metastases[3,4]. On the other hand, cystic formation caused by mucin producing metastatic tumor from colorectal cancer has only rarely been reported. Here, we report a case of mucin producing liver metastases with intrabiliary extension that was difficult to distinguish from benign cystic change.
CASE REPORT
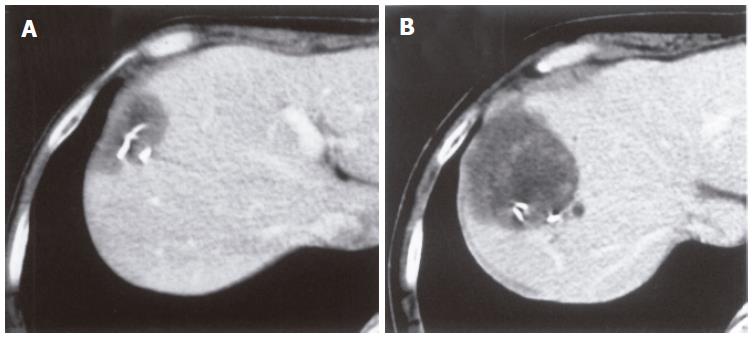
A 75-year-old man was admitted to our hospital with a liver tumor. He underwent right hemicolectomy following a diagnosis of cecal cancer in January, 1994. Histological examination of the resected specimens revealed a well-differentiated adenocarcinoma with mucinous carcinoma, which invaded the subserosal layer and metastasized to regional lymph nodes. Four years after his initial operation, he underwent partial hepatectomy of segment 8 (S8) due to a diagnosis of metastatic liver tumor on June 22, 1998. Histopathological examination of the resected specimens revealed a well-differentiated adenocarcinoma with mucinous carcinoma, similar to cecal cancer. The tumor invaded only the bile duct, but surgical margin was negative for cancer. Four months after his second operation, abdominal computed tomography (CT) revealed a low-density lesion at the cut surface of the liver. Initially, it was considered to be a postoperative collection of inflammatory fluid. Through the continuous observation using periodical CT scans, the low-density lesion gradually formed a cystic mass over two years, but little change was found in size. Two years later, the mass increased in size to approximately 5 cm in diameter (Figure 1). Cytological examination from ultrasonography (US)-guided fine needle aspiration biopsy revealed it to be class III, indicating a borderline malignancy.
Figure 1 A low-density lesion at the cut surface of the liver in December, 1998 (A) and two years later, the mass increased in size to approximately 5 cm in diameter (B).
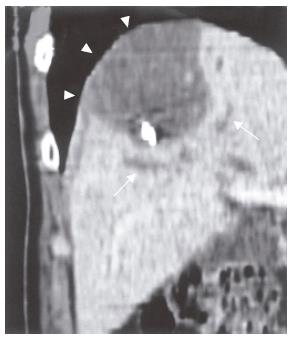
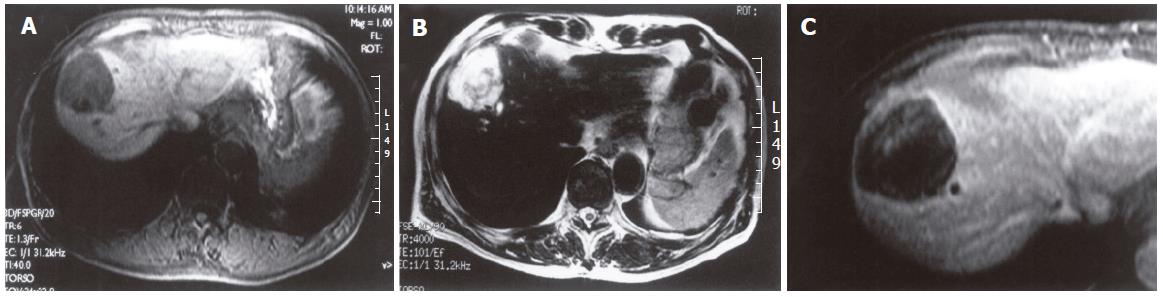
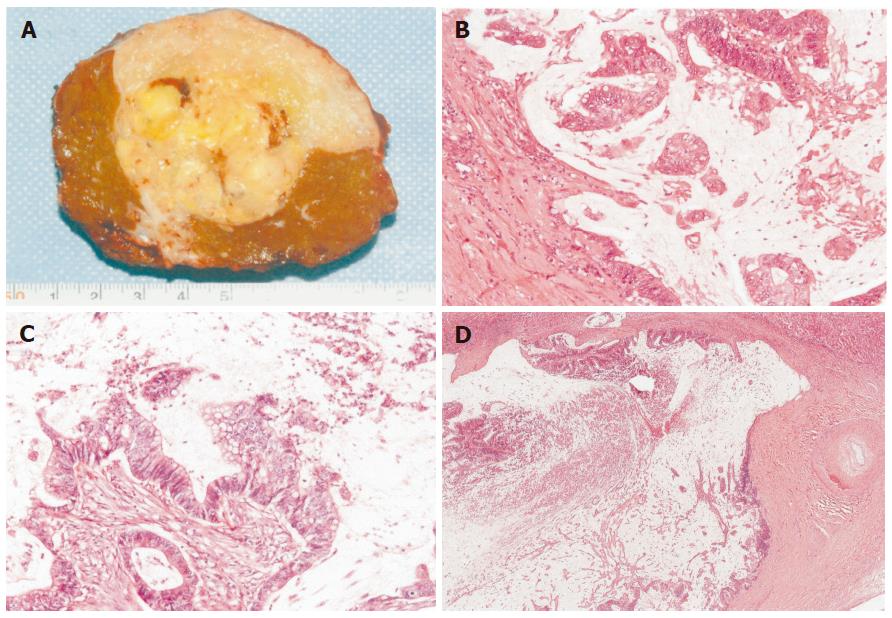
On admission, the patient exhibited no abnormal findings upon physical examination. Complete blood counts and serum chemistries were within normal limits. Among tumor markers, serum carcinoembryonic antigen (CEA) levels and alpha-fetoprotein (AFP) levels were within normal limits. The indocyanin green retention rate after 15 min (ICG R15) was 16.0%. Abdominal CT revealed a low- density cystic tumor of S8 that measured about 4 cm × 5 cm in size, with local dilatation of intrahepatic bile ducts (IHBD). The tumor was bordered by the diaphragm (Figure 2). Abdominal magnetic resonance imaging (MRI) revealed a tumor with a low intensity signal on T1-weighted images and a high intensity signal on T2-weighted images (Figures 3A and B). In addition, the edge of the tumor was slightly enhanced by gadolinium, while the inside was heterogeneous (Figure 3C). Magnetic resonance cholangiopancreatography (MRCP) revealed local dilatation of IHBD. Based upon these examinations, one of the differential diagnoses was biloma at the cut end of the previous hepatectomy. To further investigate the content of the tumor, US-guided biopsy was performed. An aspirated sample contained a small volume of yellow and clear fluid, but bile was not found. Histological examination of the biopsy specimens revealed a mucinous adenocarcinoma. Finally, the tumor was judged to be a recurrence of metastatic cancer with invasion to the IHBD. There was no evidence of metastasis in any other organs or in regional lymph nodes. The patient was considered to be a candidate for surgery. On March 13 in 2002, he underwent the third operation. When the peritoneal cavity was entered, there was no evidence of peritoneal dissemination, enlarged lymph nodes or ascites. The tumor was located in the liver in S8 adhering to the diaphragm and was about 4 cm in diameter, as measured by US. US was also performed to indicate a sufficient surgical margin of at least 15 mm. The tumor was resected using intermittent clamping of the primary branch of Glisson’s triad (Pringle’s procedure). When we cut the regional Glisson’s triad, mucinous bile was found at the cut end of the bile duct. The portal vein was intact. According to the preoperative informed consent, partial resection of the liver in sub-segment 8 and the diaphragm was performed. Histological examination of the resected specimens revealed a well-differentiated mucinous adenocarcinoma, which was consistent with the metastatic lesion from colon cancer. The tumor extended along the lumen of the biliary ducts, replacing the non-neoplastic epithelium (Figure 4). The peripheral bile ducts were obstructed by the cancer cells, and the cut end of the bile duct was positive for cancer. The tumor invaded only the abdominal side of the diaphragm and was not apparent on the thoracic side. Immunohistochemically, the tumor cells were positive for CK 20, but negative for CK7 (Figure 5). Thus, we diagnosed it as a recurrence of metastatic adenocarcinoma from cecal cancer.
Figure 2 A low-density cystic tumor in S8 with local dilatation of the IHBD (arrows).
The tumor was bordered by the diaphragm (arrow heads).
Figure 3 A low intensity signal on T1-weighted (A) and a high intensity signal on T2-weighted (B) images.
The edge of the tumor was slightly enhanced by gadolinium, while the inside was heterogeneous (C).
Figure 4 Macroscopic finding (A), well-differentiated mucinous adenocarcinoma (B) similar to cecal cancer (C), extension of tumor cells along the lumen of the biliary ducts with the non-neoplastic epitheliumreplaced (D).
(B) HE, ×100; (C) HE, ×100; (D) HE, ×18.
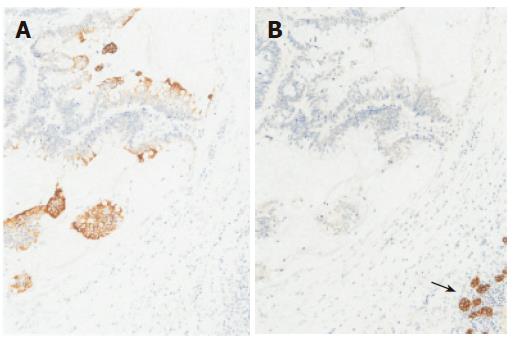
Figure 5 Immunohisotochemically, tumor cells positive for CK 20 (A), but negative for CK7 (B), normal epithelial cells of IHBD positive for CK7 (arrow).
(A) × 100; (B) × 100.
Three months after the hepatectomy, the patient exhibited a recurrence at the cut surface of the residual liver. He and his family did not hope further surgery. Although he received chemotherapy using 5-FU for the local recurrence, he died 2 years and 9 mo after the second hepatectomy.
DISCUSSION
Liver metastases occur in 25%-35 % of patients with colorectal cancer[1]. Surgical resection has become a recognized curative treatment[1,5,6]. However, the rate of intrahepatic recurrence has been reported to range from 16% to 28%[5,7], and 9%-25% of these arise at the surgical margin[8,9]. Okano et al[3] reported that 42% of patients who have undergone hepatectomy for colorectal liver metastases exhibit bile duct invasion, 12% of which exhibit macroscopic invasion. Among these metastases, an intrabiliary extension pattern is rare. Kubo et al[4] reported that 3.7% of resected colorectal liver metastases are extended predominantly along the bile duct without forming an extrabiliary mass. The prognosis of patients with macroscopic bile duct invasion is better than that of patients with microscopic intraluminal invasion, which tends to aggressively involve the Glisson’s triad[3,4].
In the present case, we initially considered the cystic lesion to be a post-operative collection of inflammatory fluid. However, the lesion turned out to be a cystic tumor including mucin produced by the remnant cancer cells. Cancer cells and mucin obstructing the IHBD, caused dilatation of the peripheral bile ducts. Microscopically, about 10% of metastatic tumors from colorectal carcinoma exhibit mucinous features[2]. Furthermore, the frequencies of incidence of localized IHBD dilatation caused by metastatic liver cancer and cystic degeneration of metastatic tumor have been reported to be 6%[10] and 2%-4%, respectively[11,12]. Therefore, the present case was considered to be a very rare case and preoperative diagnosis could not be confirmed by imaging examination alone. On the other hand, it is known that cholangiocellular carcinoma (CCC) is often accompanied with dilatation of the IHBD, because intrahepatic CCC exhibits bile duct extension, either through infiltration into the periductal tissues or appearing as a cast-like growth into the ductal lumen[13]. Furthermore, CCC sometimes exhibits mucin production. Thus, the growth of colorectal metastases with bile duct invasion can be indistinguishable from that of less aggressive CCC, and it is sometimes difficult to make a differential diagnosis histologically. To discriminate liver metastases from primary CCC, immunostaining for CK7 and CK20 can be very useful. A CK20-positive and CK7-negative pattern is highly characteristic of liver metastases from colorectal cancer, compared with most adenocarcinomas including CCC that are usually CK20-negative and CK7-positive[14-16]. In the present case, this immunohistochemical finding allowed us to make a definite diagnosis.
Our patient has survived for more than two-years, in spite of local recurrence. This may be a consequence of the less aggressive features of this tumor, which exhibits macroscopic bile duct extension[3,4]. However, because this invasion pattern tends to make the cut ends positive for cancer cells, it appears that it is necessary to examine the cut end of Glisson’s triad by stamp cytology or frozen section pathology. Actually, during the 2nd hepatectomy, mucinous bile was found in the bile duct. However, since the tumor was a metastatic colorectal cancer rather than a CCC, partial hepatectomy was a procedure of choice for us. For colorectal metastases, we usually perform partial hepatectomy with a sufficient margin rather than anatomical resection if possible. Recently, Pawlik et al[9] reported that the width of a negative surgical margin does not affect survival, recurrence risk, or site of recurrence. However, after having experienced our present case, anatomical hepatic resection may be a treatment of choice for metastatic liver tumor with bile duct extension to avoid the potential for a positive surgical margin. Further studies are necessary to develop a suitable surgical procedure for this unusual pattern of liver metastasis.