Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4866
Revised: May 1, 2006
Accepted: May 25, 2006
Published online: August 14, 2006
AIM: To explore the expansion and differentiation of hepatocytoid cell induced from myeloid mesenchymal stem cell (MSC) in vitro, in order to find suitable resource of hepatocytes for bioartificial liver or liver transplantation.
METHODS: The rat myeloid MSC was isolated and divided into three groups which were cultured by Friedensteion method, and then were induced by culture fluid, culture fluid plus cholestatic serum and culture fluid plus hepatocyte growth factor (HGF), respectively. Hepatocytoid cell as well as expression of CK18 and AFP was observed by immunohistochemistry.
RESULTS: After the induction for 21 d, hepatocytoid cell was observed, and its expression of CK18 and AFP was detected by immunohistochemistry in MSC cultured with cholestatic serum. Furthermore, on the 35th d, albumin mRNA was expressed in the cell, suggesting the inducing effect was similar to that by HGF.
CONCLUSION: Rat myeloid MSC can differentiate into hepatocyte lineage under appropriate condition. This method is easy to operate.
- Citation: Li W, Liu SN, Luo DD, Zhao L, Zeng LL, Zhang SL, Li SL. Differentiation of hepatocytoid cell induced from whole-bone-marrow method isolated rat myeloid mesenchymal stem cells. World J Gastroenterol 2006; 12(30): 4866-4869
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4866.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4866
Over the past few years, great progress has been made in the research of stem cell. It has been proved that some stem cells in bone marrow can differentiate into various kinds of cells. Therefore, through culturing myeloid stem cells in vitro, and providing suitable conditions, these cells can be induced to generate and differentiate into other cells. There have been some reports that application of cytokines such as hepatocyte growth factor (HGF), fibroblast growth factor-4 (FGF-4) might promote myeloid stem cells to proliferate and differentiate into hepatocytoid cell. However, the cytokines are costly. This study was to explore a convenient way to induce myeloid mesenchyme stem cell (MSC) into hepatocytoid cell in vitro.
One-month-old Wista rats were provided by the Animal Center of Tongji Medical College, Huazhong University of Science and Technology. Rat recombinant hepatocyte growth factor (HGF) (R&D Co.), Dulbecco’s minimum essential medium (DMEM) (Gibco), fetal bovine serum(FBS, Hyclone), N-2-hydroxyethylpiperazine-N’-2’-ethanesulfonic acid (HEPES), galactose solution of insulin, transferrin and selenite(ITS) (Sigma), micotinamide, trypsinase(Gibco), proline(Wuhan Lingfei Bio. Co.), alpha fetoprotein(AFP) monoclonal antibody (MoAb) (Santa Cruz Co.), cytokinin 18 (CK18) MoAb, biotin labeled Ab, diaminobenzidine(DAB), streptavidin peroxidase (S-P) (Beijing Zhongshan Co.), deoxy-ribonucleoside triphosphate (dNTPs), Pyrotest Taq enzyme(Bao Bio Ltd. Co.), Bio-11-dUTP (Huamei Biotech Co), Deion formamide, salmon sperm DNA(Sigma), fluorescein isothiocyanate (FITC) labeled antibiotin(Boshide) and albumin primer 5’ATACACCCAGAAAGCACCTC3’, 5’CACGAATTGTGCGAAGTC AC3’ (Shenggong Co) were prepared.
Isolation and culture of myeloid MSC: According to the literature[1], rat femurs were separated under aseptic condition. The myeloid cell, after douched and blew out from the bone with culture fluid (5 mL DMEM + 10% FBS), was added to the culture fluid to make cell suspension. Then the culturing was begun with the supplementation of fluid of DMEM, 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin, at 37°C, in 50 mL/L CO2 environment. On the third day non-adherent cell was removed and the remaining cells were allowed to grow. On the 10th d the confluent cells were trypsinized and passaged. When the laminating cell with typical cellular appearance was observed, 0.25% trypase was added for digestion for 5-10 min and then the whole medium was added to terminate the digestion. When the innoculation density was 1 × 104/cm2, the nutrient medium was added and the serial subcultivation was begun. The above procedure was repeated to passage for every 3-4 d.
MSC differentiation potency detection: The third generation MSC was inoculated onto 6-well culture plate (density: 5 × 104/mL), and the induction culture fluid (0.05 g/L vitamin C, 40 ng/mL dexamethasone, 10 mmol/L β sodium glycerophosphate) was added. Then the cell was passaged for every 4 d. On the 15th d autoclaved coverslips were put into the media and 3 d later the coverslips were taken out and were stained by alkaline phosphatase.
Cholestatic serum preparation: Male rats with weight of 400 g were anesthetized with Pentothal and their abdomens were degermed. After the abdominal cavities were opened, their choledochuses were ligated and then the bellies were sutured. Given normal feed for 10 d, those rats were dissected again at abdomen to obtain blood from inferior caval vein. The serum was separated and preserved in refrigerator at -40°C.
Group division: Three groups, including MSC culture group (only with culture fluid), MSC cultured with cholestatic serum (5%) group and MSC cultured with HGF (0.5 mg/mL) group, were divided. The culture fluid composed of DMEM, 10% FBS, 15 mmol/L HEPES, 10 mmol/L niacinamide, 1 mmol/L vitamin C, 10-7 mol/L dexamethasone, 1 mg/mL galactose, 30 μg/mL proline, ITS and antibiotics. All the cells were inoculated onto 6 well culture plate and allowed to creep on the plate coverslips custodited with polylysine (0.025%). The morphology of the cell was observed on the 7th, 14th, 21st and 35th d according to the literature [1] and during those days the coverslips were taken out and fixed with paraform (4%) for 30 min.
Detection methods: The expression of CK18 and AFP by the cells was detected by immunohistochemistry and S-P method, with PBS instead of 1st-antibody in negative control. The albumin expression was detected by in situ hybridization. Biotin random primer labeling method was applied as albumin labeling probe.
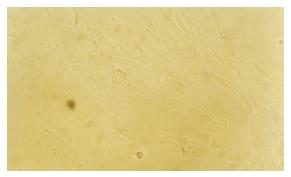
Growth condition of MSC culture group: The primary cells adhered to the wall after cultured for 48 h, and the adhered cells increased gradually afterward. After 3 passages, on the 14th d, the cells showed uniform morphology (Figure 1) like fibroblast and grew as grass bunch or whirlpool.
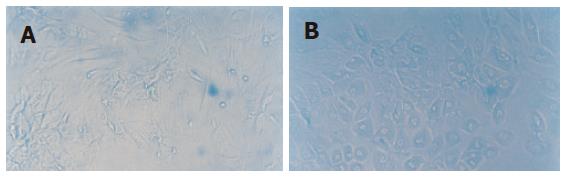
Growth condition of MSC cultured with cholestatic serum group: From the 35th d, the cell shape changed from fusiform to polygon and the proportion of the polygon cell increased with time. Meanwhile, the cell proliferation was also slowed. These phenomena appeared first in the periphery of the cell clone, and gradually extended into the whole cell clone (Figure 2).
Growth condition of MSC cultured with HGF group: The characteristics of the cell in this group were similar to that in MSC cultured with cholestatic serum group (Figure 3).
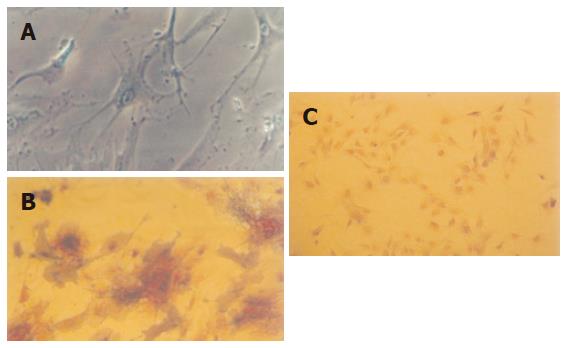
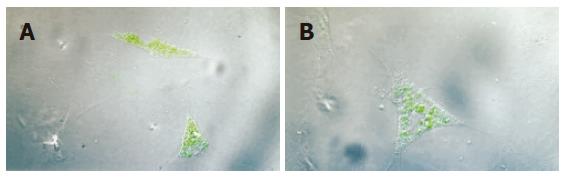
After induced by vitamin C, dexamethasone and β sodium glycerophosphate, respectively, the cells gradually changed to different morphological forms, like triangle, fusiform and polygon. With the prolongation of culturing time, the cell population increased and cell volume enlarged. On the 15th d, the cell endochylema was full and the cells connected with each other with ecphyma. Alkaline phosphatase staining showed positive (Figure 4). This verified that the cells isolated were MSCs.
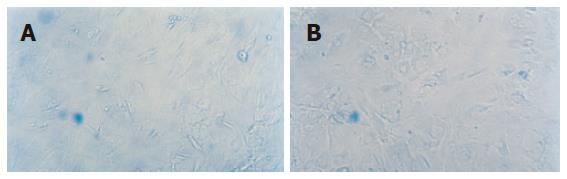
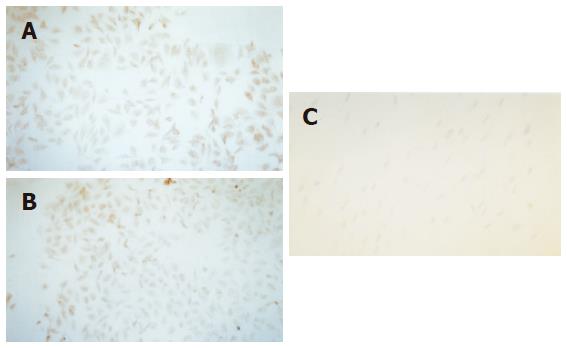
Expression of AFP by the cell was observed on the 14th d and expression of CK18 was detected on the 21st d in MSC cultured with cholestatic serum. And these two manifestations could still be observed on the 35th d. As comparison, MSC cultured with HGF also showed the same distinction but the other groups did not show the feature (Figure 5).
Albumin was expressed both in MSC cultured with cholestatic serum and MSC cultured with HGF groups, but the former was more significant (Figure 6).
There are two approaches to isolate MSCs including whole-bone marrow method[2] and density gradient centrifugation method[3]. The former, by which we employed in this experiment, is to purify MSCs by removing the non-attached cells when culture fluid is changed, according to the different adhering capabilities of stem cell. The latter is by adherently culturing mononuclear cell, based on the conception that different cell component in myeloid has different density. Nowadays, some new techniques have been developed to isolate MSCs, such as flow cytometry and immunomagnetic beads method. However, those new methods, being costly and technically difficult, have defect of inhibiting proliferation after sorting. So we preferred the whole-bone marrow method to purify MSC in this experiment, although the cells were of inadequate uniform, and heterogeneous in population.
Up to now, there has been no agreement on the phenotypical characterization of a “pure” population of human MSC despite the panoply of surface antigens reported to be expressed on MSC[4-6]. Therefore, identification of MSC has been a puzzle. In 1999, Pittenger et al[4] isolated MSC from human flank bone marrow and proved that MSC is pluripotent stem cell in that it could be induced into osteoblast, chondrocyte and lipocyte. Thus, through analyzing the differentiation phenotype during culturing, we could identify MSC. In our experiment, it was observed that osteoblast cytoplasm was basophilic and AKP stain positive. Because the osteoblast was the unique cell in differentiated MSC population, it proved the existence of MSC.
Recently, Verfaillie et al[7] demonstrated that multipotent adult progenitor cells(MAPCs) from rat, mouse and human could differentiate into functional hepatocytes. They detected that the cells could synthesize urea, albumin and amylon, possess activity of cytochrome P450, adsorb α-low-density lipoprotein and did not have carcinogenesis process. It was not clear whether the MSCs we isolated included MAPCs; nevertheless, the cell could excrete CK18, AFP and albumin, which functions were similar to that of hepatocytes. AFP and CK18 are the characteristic proteins expressed during hepatocyte development. In addition, the method we used was convenient, easy to perform, not expensive and the cell still maintained cleavage and differentiation activity after numerous generations, thus it has wide application value in future.
Many investigations[8] have revealed that bone marrow stem cell (BMSC) or purified hepatic stellate cell (HSC) can differentiate into hepatocytes. To find a suitable inducing condition has become a research hotspot lately. From the results of our study, it could be inferred that the induction of MSC by the cholestatic serum was probably through humoral signal pathway. The likely mechanism is as follows: after the choledochus was ligated, acute hepatic necrosis developed, and the liver was atrophy with a large amount of ascites in abdominal cavity; then hepatocyte growth promoters such as HGF, EGF and FGF were produced; and the cholestatic serum containing those cytokines might promote differentiation of MSC.
The concentration of cholestatic serum in our study, 50 mL/L, with coincidence to the literature[9,10] had a good effect to induce hepatocytoid cell to secrete CK18, AFP and albumin. But the constituent of cholestatic serum, being complicated and with individual variation could not be detected precisely; therefore it needs further exploration to define ingredients of the cholestatic serum, and moreover, to verify the function of synthesis and secretion, as well as metabolism and non-carcinogenesis nature of the induced hepatocytoid cell. Bone marrow stem cells represent a safe and accessible source of stem cells, and the use of MSCs provides a potential treatment approach for severe liver diseases.
| 1. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 665] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-272. [PubMed] |
| 3. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3925] [Article Influence: 163.5] [Reference Citation Analysis (5)] |
| 4. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15370] [Article Influence: 569.3] [Reference Citation Analysis (2)] |
| 5. | Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol J. 2003;4:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1353] [Article Influence: 54.1] [Reference Citation Analysis (7)] |
| 7. | Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 750] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 9. | Cai YF, Zhen ZJ, Min J, Fang TL, Chu ZH, Chen JS. Selection, proliferation and differentiation of bone marrow-derived liver stem cells with a culture system containing cholestatic serum in vitro. World J Gastroenterol. 2004;10:3308-3312. [PubMed] |
| 10. | Yamazaki S, Miki K, Hasegawa K, Sata M, Takayama T, Makuuchi M. Sera from liver failure patients and a demethylating agent stimulate transdifferentiation of murine bone marrow cells into hepatocytes in coculture with nonparenchymal liver cells. J Hepatol. 2003;39:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Zhu LH E- Editor Ma N