Published online Aug 7, 2006. doi: 10.3748/wjg.v12.i29.4660
Revised: January 28, 2006
Accepted: February 18, 2006
Published online: August 7, 2006
AIM: To study CD34, CD105, inducible nitric oxide synthase (iNOS), endogenous nitric oxide synthase (eNOS), and hypoxia-inducible factor 1 (HIF-1) α expression in human colorectal carcinomas.
METHODS: The tissue microarrays (TMAs) were made up of 80 cases of colorectal carcinoma and 80 cases of non-neoplasm colorectal mucosa. The expression of CD34, CD105, NOS and HIF-1α was detected by immunohistochemistry (S-P).
RESULTS: iNOS and HIF-1α expression in colorectal carcinoma was significantly higher than in non-neoplasm colorectal mucosa (χ2 = 43.166, P < 0.01; χ2 = 10.4278, P < 0.01); eNOS expression in colorectal carcinoma was significantly lower than in non-neoplasm colorectal mucosa (χ2 = 11.354, P < 0.01). The expression of iNOS correlated with differentiation (χ2 = 18.141, P < 0.01), invasive depth (χ2 = 4.748, P < 0.01), and Micro vessel density (MVD) (t = 2.327, P < 0.05). The expression of HIF-1α was correlated with infiltrating depth (χ2 = 4.397, P < 0.05), Duke’s staging (χ2 = 4.255, P < 0.05), and MVD (t = 2.272, P < 0.05). No correlation was found in eNOS expression.
CONCLUSION: Over-expression of iNOS and HIF-1α in colorectal carcinoma is correlated with the biological character MVD.
- Citation: Yu JX, Cui L, Zhang QY, Chen H, Ji P, Wei HJ, Ma HY. Expression of NOS and HIF-1α in human colorectal carcinoma and implication in tumor angiogenesis. World J Gastroenterol 2006; 12(29): 4660-4664
- URL: https://www.wjgnet.com/1007-9327/full/v12/i29/4660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i29.4660
Angiogenesis is an important step in the growth and metastasis of solid malignant tumors. To adapt to hypoxia, angiogenesis is essential for tumor growth. Tumor must over-express genes that encode angiogenic factors to induce new blood vessels[1]. A number of angiogenic factors have been found, and there is interrelation among them, which has been widely researched. Nitric oxide synthase (NOS) and hypoxia-inducible factor 1 (HIF-1) α are two of these factors which may be correlated with angiogenesis. To screen out angiogenic factors is time-consuming and expensive. Tissue microarray (TMA) brings to pathologists a completely new method to solve this problem. TMA has many excellent features such as small volume, high information content, and assembling differently according to different needs. A number of results can be obtained through one experiment[2], which reduces research time and improves efficiency greatly. Moreover, the most important advantage is a decrease of experimental error, because TMA can make all samples under the same experimental condition. To study NOS and HIF-1α as angiogenic factors, we used immunohistochemistry to detect the expression of CD34 and CD105 and the expression of inducible NOS (iNOS), endogenous NOS(eNOS), and HIF-1α in human colorectal carcinomas. TMAs consisting of 80 colorectal carcinoma and 80 non-neoplasm colorectal mucosa were employed to accomplish these studies.
Eighty specimens were collected from 80 patients with colorectal carcinoma (median age 62.2 ± 5.9 years, range 29-88 years) who were operated in Qingdao Municipal Hospital (Shandong, China) from Oct 2003 to Mar 2004. Carcinoma and non-neoplasm colorectal mucosa (located at least 8 cm away from the margins of cancers) were taken from each sample. There were 6 cases of well-differentiated adenocarcinoma, 59 cases of moderately differentiated adenocarcinoma and 15 cases of poorly differentiated adenocarcinoma. Among them, 30 cases had regional lymph node metastasis and 12 cases had distant metastasis. None of the patients received any chemotherapy or radiation therapy before surgical operation. The pathological diagnosis was made on the basis of the size, infiltrating depth, histological grade of the carcinoma, and lymph node and distant metastasis. The grading standard was in accordance with that established in the book Practical and Surgical Pathology[3].
HE staining sections of all samples were observed firstly, and typical lesion positions were marked. Then the corresponding positions to these lesions were marked on paraffin wax. The empty TMA paraffin wax (45 mm × 28 mm× 7 mm) was holed through Tissue Arrayer (each diameter was 2 mm), and the tissues taken from marked positions were put in these holes tidily (each diameter was also 2 mm). The process was repeated for many times and the TMA paraffin wax was ready (Figure 1A). A different kind of tissue was put in it as a marker, which was kidney tissue in this experiment. Each TMA paraffin wax was sliced into 4 μm thick sections.
The expression of CD34, CD105, NOS and HIF-1α was stained by immunohistochemistry (S-P). The reagent kits were purchased from Fuzhou Maxim Co. Micro vessel density (MVD) was counted with different vessel markers CD34 and CD105 respectively. CD34 and CD105 antibodies were supplied from Neomarker Company, and working consistence was 0.2 mg/L, and 0.4 mg/L. iNOS and eNOS multi-clonal antibody was purchased from Neomarker Company; working consistence was 20 mg/L, and 20 mg/L, respectively; multi-clonal HIF-1α antibody was from Santa Cruz Company; working consistence was 0.8 mg/L. Homogenous animal IgG that contained the same protein quantity was used instead of first antibody in negative control.
NOS and HIF-1α staining was classified into two groups. Positive group includes: +++, most carcinoma cells were stained with a very strong intensity, and distributed in clusters; ++, a large number of carcinoma cells were stained with a moderate intensity, and distributed in clusters occasionally; +, a few carcinoma cells were stained with a slight intensity. Negative group is defined as: -, no staining of carcinoma cells.
Counting of MVD in carcinoma tissues was in accordance with Weidner’s standards with a minor modification. The sections were searched for the hot spots rich in vessels, which were located in or near the area of tumor tissues under a low power microscope (100 × ). MVD was counted under a high power (400 × ) microscope according to the standards that any stained endothelial cell or cells were identified as an independent vessel. These vessels must be clearly separated from each other. However, apparent vasa or vasa with red blood cells could be regarded as vessels. Three different HP vision fields were chosen on each of the sections. Stained vessels were counted by two pathologists under multi-ocular microscope simultaneously. The results were averaged as the relative value of the amount of vessels per unit area.
The difference of MVD between CD105 and CD34 staining was analyzed with paired sample t-test. The relation between MVD and expression of NOS and HIF-1α was analyzed with t-test. Pearson-chi-square test was used for statistical analysis of the relation between clinical-pathological parameters and expression of NOS and HIF-1α.
Every TMA section consisted of 36-46 samples, which were arrayed orderly. The shape of these samples was rounded or elliptoid, and a few samples were crimpled or lost (Figure 1B). After TMA wax was sliced consecutively, one section was picked out for HE staining to make sure that the tissue still kept its histological feature. In fact, almost all samples could keep their histological feature in TMA wax which total depth was about 3 mm. Adjacent sections that contained all samples were selected. After antigen retrieval and IHC staining, 1-2 samples fell off from the sections. Therefore, the number of available samples was 78, and the availability was 97.5%.
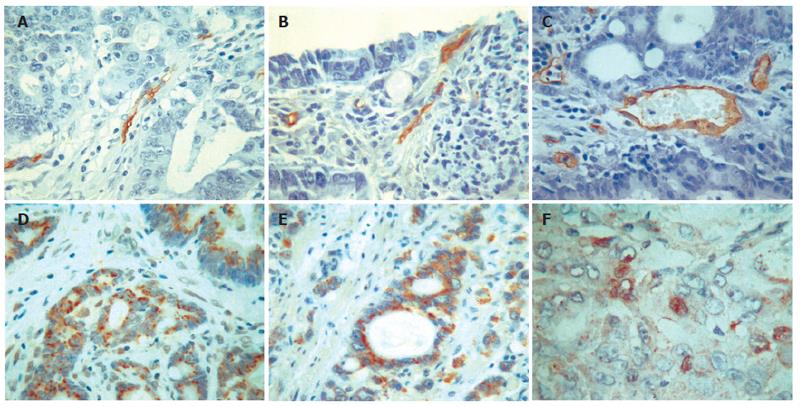
CD105 was mainly expressed in cytoplasm and plasmalemma of newborn endothelial cells. These positive cells were shown as one cell or a group of cells, and most of them ranged in a line or an irregular goblet while a few of them formed lumen. CD105 was weakly expressed or absent in native blood vessels with thick walls or large lumina (Figure 2A). The vessels with positive CD34 staining formed lines or had obvious lumina. Endothelial cells of many large vessels with thick walls and smooth muscles showed positive staining, which was distinct from the sections of CD105 staining (Figure 2B and C). t-test showed that MVD by CD34 staining was significantly different from that by CD105 staining (27.5 ± 10.3 vs 14.0 ± 5.8, t = 4.782, P < 0.01).
The positive rate of iNOS was 78.2% (61/78) in colorectal carcinoma, and 25.6% (20/78) in non-neoplasm mucosa of colorectal tissue. The difference between them was significant (χ2 = 43.166, P < 0.01). The positive granules of iNOS were mainly located in cytoplasm diffusely (Figure 2D). The positive rate of iNOS decreased with the descending of differentiation grade. Most ring carcinoma showed negative expression. The expression of eNOS showed positive or weak positive in non-neoplasm mucosa of colorectal tissue. The positive rate was 60.3% (47/78). The positive granules also located diffusely in cytoplasm, and some expressed in cell nucleus (Figure 2E). The expression of eNOS in colorectal carcinoma was 33.3% (26/78), which was statistically different from that in non-neoplasm mucosa of colorectal tissue (χ2 = 11.354, P < 0.01). The positive rate of eNOS also decreased with the descending of differentiation grade. (Table 1)
| Pathology | n | iNOS | eNOS | HIF-1α |
| Histological grade | ||||
| Well differentiation | 6 | 5 (83.3)b | 3 (50) | 2 (33.3) |
| Moderate differentiation | 58 | 51 (87.9) | 21 (36.2) | 43 (74.1) |
| Poor differentiation | 14 | 5 (35.7) | 2 (14.3) | 9 (64.3) |
| Infiltrating depth | ||||
| Serous membrane (-) | 9 | 4 (44.4)d | 2 (22.2) | 3 (33.3)a |
| Serous membrane (+) | 69 | 57 (82.6) | 24 (34.8) | 51 (73.9) |
| Lymph node metastasis | ||||
| (+) | 29 | 24 (82.8) | 11 (37.9) | 23 (79.3) |
| (-) | 49 | 36 (77.9) | 15 (33.3) | 31 (63.3) |
| Distant metastasis | ||||
| (+) | 12 | 11 (91.7) | 5 (41.7) | 11 (91.7) |
| (-) | 66 | 50 (75.8) | 21 (31.8) | 43 (65.2) |
| Duke’s stage | ||||
| A, B | 45 | 35 (77.8) | 18 (40.0) | 27 (60)c |
| C, D | 33 | 26 (78.8) | 8 (24.2) | 27 (81.8) |
The positive rate of HIF-1α was 69.2% (54/78) in colorectal carcinoma, and 43.6% (34/78) in non-neoplasm mucosa of colorectal tissue (χ2 = 10.4278, P < 0.01). The positive granules mainly located in cell nucleus and cytoplasm (Figure 2F). A small number of HIF-1α granules were expressed in inflammatory cells in the stroma of tumor.
Pearson-Chi-square test showed that the expression of iNOS was correlated with differentiation (χ2 = 18.141, P < 0.01), and infiltrating depth (χ2 = 4.748, P < 0.01). The expression of eNOS had no significant difference in different groups. However, the expression of HIF-1α was correlated with infiltrating depth (χ2 = 4.397, P < 0.05), and Duke’s staging (χ2 = 4.255, P < 0.05). MVD of iNOS positive group was significantly higher than that of the negative group (14.3 ± 6.1 vs 10.6 ± 4.0, t = 2.327, P < 0.05); HIF-1α staining showed the same result (15.2 ± 5.4 vs 11.5 ± 4.3, t = 2.272, P < 0.05), while the MVD between the two groups of eNOS staining had no significant difference (13.8 ± 5.4 vs 11.9 ± 4.7, t = 1.523, P > 0.05).
Angiogenesis refers to the formation of new blood vessels from native blood vessels, which is very important to the growth and invasion of malignant solid tumors[4]. In 1971, Folkman firstly put forward the viewpoint that the growth of tumor depends on angiogenesis[5]. In tumor microenvironment angiogenesis needs many steps, which is a continuous process. The characteristics of micro vessels in tumors are different from that developed under normal physiological conditions. In fact, MVD has been extensively investigated and was found to be a useful prognostic marker in many types of cancers[6].
At present, most researchers use immunohistochemistry (IHC) to study new blood vessel in tumors. Therefore it is necessary to select an appropriate marker of endothelial cells[7]. Previous reports have demonstrated that MVD-CD34 is a better prognostic marker than MVD assessed by many other endothelial markers, such as von Willebrand factor (vWF), CD31. However, CD34 is a pan-endothelial cell marker, thus it is difficult to distinct newborn vessels from native vessels, and it may not be able to reflect the exact angiogenesis activity in a tumor. According to some previous reports, CD105 is an endothelial marker that appears to react only with the endothelial cells in the newly formed vessels, and in particular, the immature tumor blood vessels[8]. Based on our study, we suggest that CD105 has a higher rate of positivity and stronger reactivity on the endothelial cells of new blood vessels[9]. Therefore CD105 is a better marker for the endothelial cells of new blood vessels. In addition, CD105 monoclonal antibody in the experiment was suitable for paraffin sections. It overcame the limit that usual antibody is only used in frozen sections. Because frozen sections were more difficult to observe than paraffin sections, it led to the inconvenience for MVD counting under microscope. Using this antibody, we saved time and materials, and obtained better staining effect. Moreover, the result of the experiment was more reliable and accurate.
NOS includes eNOS and iNOS, which have different functions. eNOS is involved in many physiological functions of neural system, such as regulating the angioectasis and smooth muscle hyperplasia. iNOS expression in human diseases has long been investigated. NO production by iNOS in tumor infiltrating macrophages may be part of their anti-tumoral cytotoxic potential[10]. On the other hand, the expression of iNOS in endothelial cells of tumor vessels and in cancer cells itself supports the assumption that the cancer uses this form to regulate the tumor vascularisation and blood flow. eNOS exists in normal condition and only produce a slight number of NO, while iNOS is induced by activated cells to produce more NO which can promote the construction of tumor vessels. NO has been found to be a mediator of angiogenesis and blood flow[11,12].
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric transcriptional factor composed of two basic-helix-loophelix (bHLH)-PAS α and β subunits. HIF-1α is the unique, O2-regulated subunit that determines HIF-1 activity[13]. A series of genes and proteins that may increase the survival of tumor cells under hypoxia conditions, including vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), iNOS, platelet-derived endothelial growth factor (PDEGF), glucose transporter 1, lactate dehydrogenase, erythropoietin and nitric oxide synthase gene, are regulated by HIF-1α. Thus, HIF-1α may play important roles in tumor progression. Furthermore, HIF-1α is a critical factor of transcription in the pathway of vascular growth signals. It can activate many genes of angiogenic factors, which can lead to angiogenesis. Most studies about the effects of HIF-1α on the neovascularization in malignancies have been done in vitro or in animal models. It has been confirmed that in many cancer cell lines, hypoxia can induce the expression of some proangiogenic factors, including VEGF, IGF, and PDEGF, through HIF-1α-dependent manner. It has also been proven through the method of gene knockout that the loss of HIF-1α may significantly suppress the growth of tumors including glioblastoma and malignant teratoma and most importantly, it may decrease the neovascularization of those malignancies. This provides a new approach for the treatment of tumor[14].
Tissue chip is also called tissue microarray, which is a special biological chip invented by Kononen in 1998[15]. Its principle is to remove hundreds of minute tissues from different primary blocks and place them into one arrayed block with high density. Tissue microarray is suitable for all analyses in situ such as antibody staining, immunohistochemistry, fluorescence in situ hybridization, Western blot. Thus we can investigate the different expression of tissues either at DNA and RNA level or protein level. Tissue microarray is characterized with small bulk and high-throughput, which can produce many results through one experiment[2,16]. It can decrease experiment errors, greatly improve the efficiency of histopathological study, save experimental materials and reagents, and at the same time make the results more reliable and comparable[17-19]. Bubendorf et al[20] made a large amount of tissue microarrays of tumor, in which ten blocks had 4788 samples from 130 kinds of tumor. These microarrays can be used to analyse the difference of newly found genes between normal tissues and tumor, from which we can acquire reliable results. By using the same method, Fernebro et al[21] also found that Ki67 positive cell index of rectal carcinoma was 0.5-1.0 (average 0.85) by the method of microarray, being not significantly different from that of routine examination method (0.54-1.0, average 0.81); moreover, both methods had the same result on p53 (average 75%). These indicate that we could get reliable and appropriate results with tissue microarray for IHC staining. We have made tissue microarrays consisting of 80 cases of colorectal carcinoma, each of which has 40-50 samples, arranging orderly. Its shape is round or oval, with few crimples and it does not fall off easily. By using tissue microarrays, it is feasible to examine clinical tissue samples on large-scale and with high efficiency, which is rapid, convenient, economical and precise.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bai SH
| 1. | Fox SB, Gatter KC, Leek RD, Harris AL, Chew KL, Mayall BH, Moore DH 2nd. More about: Tumor angiogenesis as a prognostic assay for invasive ductal breast carcinoma. J Natl Cancer Inst. 2000;92:161-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Zhou XG, Zhang JS, Zhang XP. Tissue microarray. Zhonghua Binglixue Zazhi. 2002;31:70-72. |
| 3. | Chen ZN. Practical and Surgical Pathology. 1st ed. Shanghai: Shanghai Medical University Press 1997; . |
| 5. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5974] [Article Influence: 108.6] [Reference Citation Analysis (1)] |
| 6. | Newman PJ, Berndt MC, Gorski J, White GC 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 721] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Emoto M, Iwasaki H, Mimura K, Kawarabayashi T, Kikuchi M. Differences in the angiogenesis of benign and malignant ovarian tumors, demonstrated by analyses of color Doppler ultrasound, immunohistochemistry, and microvessel density. Cancer. 1997;80:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Schimming R, Marmé D. Endoglin (CD105) expression in squamous cell carcinoma of the oral cavity. Head Neck. 2002;24:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127-32136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392-4396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 565] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 11. | Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 903] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 12. | Dulak J, Józkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wójtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Sutherland RM. Tumor hypoxia and gene expression--implications for malignant progression and therapy. Acta Oncol. 1998;37:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 783] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 15. | Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 2995] [Article Influence: 107.0] [Reference Citation Analysis (1)] |
| 16. | Yang J, Wang KM, Su BS. Appliance of tissue microarray for in situ hybridization. Linchuang Yu Shiyan Binglixue Zazhi. 2002;18:344. |
| 17. | Gulmann C, Loring P, O'Grady A, Kay E. Miniature tissue microarrays for HercepTest standardisation and analysis. J Clin Pathol. 2004;57:1229-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Gancberg D, Di Leo A, Rouas G, Järvinen T, Verhest A, Isola J, Piccart MJ, Larsimont D. Reliability of the tissue microarray based FISH for evaluation of the HER-2 oncogene in breast carcinoma. J Clin Pathol. 2002;55:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Pacifico MD, Grover R, Richman P, Daley F, Wilson GD. Validation of tissue microarray for the immunohistochemical profiling of melanoma. Melanoma Res. 2004;14:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (7)] |
| 21. | Fernebro E, Dictor M, Bendahl PO, Fernö M, Nilbert M. Evaluation of the tissue microarray technique for immunohistochemical analysis in rectal cancer. Arch Pathol Lab Med. 2002;126:702-705. [PubMed] |