Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4586
Revised: January 12, 2006
Accepted: January 24, 2006
Published online: July 28, 2006
Endoscopic visualisation of gastric atrophy is usually not feasible with conventional endoscopy. Magnifying endoscopy is helpful to analyze the subepithelial microvascular architecture as well as the mucosal surface microstructure without tissue biopsy. Using this technique we were able to describe the normal gastric microvasculature pattern and we also identified characteristic patterns in two cases of autoimmune atrophic gastritis.
- Citation: Anagnostopoulos GK, Ragunath K, Shonde A, Hawkey CJ, Yao K. Diagnosis of autoimmune gastritis by high resolution magnification endoscopy. World J Gastroenterol 2006; 12(28): 4586-4587
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4586
Endoscopic visualisation of gastric atrophy is usually not feasible with conventional endoscopy. Except for the absence of rugae and visible vessels in the gastric corpus, macroscopic features, as observed during gastroscopy, are of very limited value in the evaluation of the presence of gastric atrophy[1]. Although histology may be considered as a gold standard for detection of gastric atrophy, neither the original nor the revised version of the Sydney system reliably identifies more than half of the cases in patients with confirmed atrophy[2].
Magnifying endoscopy is a helpful procedure to analyze the subepithelial microvascular architecture as well as the mucosal surface microstructure without tissue biopsy[3]. Using this technique we were able to observe the normal gastric microvasculature pattern and we also identified characteristic patterns in two cases of autoimmune atrophic gastritis.
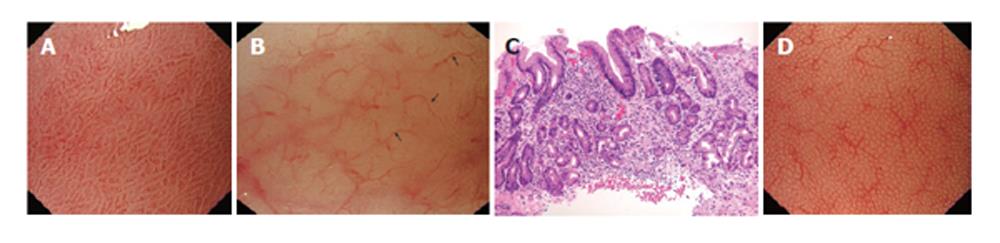
Two male patients (aged 53 and 62 years), underwent upper gastrointestinal endoscopy due to anaemia and low serum B12 levels. Endoscopy was performed using the GIF-Q240Z (Olympus, Keymed, UK) high-resolution magnifying endoscope (× 115 on a 20’’ screen, 7.9 μm resolution). Prior to endoscopy, a soft black hood was mounted on the tip of the scope, to enable the endoscopist to fix the focal distance at 2 mm between the tip of the scope and the gastric mucosa. Ordinary endoscopic findings were normal, however, magnified observation of the gastric mucosa showed normal gastric antral microvasculature (coil-shaped subepithelial capillary network (SECN) pattern) (Figure 1A), with disappearance of the normal honeycomb-like SECN and irregular collecting venules and areas of tubular structures in the gastric body mucosa (Figures 1B). Targeted biopsies from the gastric body showed glandular atrophy, intestinal metaplasia and inflammatory infiltrate in the gastric corpus, sparing the antrum, as in autoimmune gastritis (Figure 1C). On the other hand, the histological findings in the biopsied specimens from the antral mucosa showed no pathological changes. Histology showed no Helicobacter pylori micro-organisms. Serum anti-parietal cell antibodies were positive.
Autoimmune gastritis is characterized by autoimmune destruction of fundic and body glands. The marker for the most severe, end-stage form of diffuse corporal atrophic gastritis is pernicious anemia[4]. However, no endoscopic findings specific for this entity have been reported.
The microvascular pattern of human gastric mucosa has recently been investigated[5-7]. The normal gastric body and fundus mucosal microvessels show two major components: (1) subepithelial capillaries; and (2) collecting venules. Polygonal loops of subepithelial capillaries surround the neck of gastric pits. These loops form a honeycomb-like SECN and converge onto mucosal collecting venules that drain down into the submucosa (Figure 1D). In the antral mucosa, the normal subepithelial capillaries form a coil-shaped network, while collecting venules are rarely seen.
Yao et al have demonstrated that the resolution of the GIF-Q240Z gastroscope is 7.9 μm. Since the minimal diameter of subepithelial capillaries in the gastric mucosa, as described in anatomic studies, is 8 μm, this endoscope is ideally suited to visualize microvessels and capillaries[6].
Nakagawa et al have recently studied the usefulness of magnifying endoscopy for the diagnosis of H pylori-induced histopathologic gastritis[8]. The observed morphology of collecting venules was divided into the 3 patterns: (1) regular (R), which had the qualities of regularity in venules size, visible second or third order branches, and a uniform distance between venules; (2) irregular (I), which had the qualities of irregularity in size, inability to observe second or third order branches, and a lack of a uniform distance between the collecting venules, with venules sometimes fused to adjacent venules and sometimes lying horizontally; and (3) obscured (O), in which no collecting venules were visible. Observation of an R pattern indicates an absence of H pylori infection and histopathologic gastritis. Observation of an O or I pattern indicates the presence of histopathologic gastritis from H pylori infection, and an I pattern suggests the presence of gastric mucosal atrophy. We have also shown that in cases of gastric atrophy associated with H pylori infection, the normal honeycomb-like subepithelial capillary network (SECN) in gastric body disappears and the collecting venules become irregular[9].
The magnified endoscopic findings of diffuse atrophy in gastric body mucosa and normal antrum associated with autoimmune gastritis have not yet been described. In our cases, the magnified views in gastric antrum revealed a normal coil-shaped subepithelial capillary network. On the other hand, in gastric body mucosa we demonstrated loss of the normal honeycomb-like SECN pattern with irregular collecting venules, suggestive of corporal gastric atrophy, as well as areas with tubulovillous structures as seen in intestinal metaplasia. These magnified endoscopic findings were constantly detected in all parts of the gastric corpus that were examined. These patterns are suggested to be characteristic of autoimmune gastritis and were confirmed by histology.
In summary, it seems that irregular collecting venules can be recognised both in autoimmune- and H pylori-associated gastric atrophy. The dissemination of this finding can be used to help differentiate between these two types of gastritis. Gastric body predominant atrophy visualised by magnifying endoscopy, can be very useful in making a precise diagnosis of autoimmune gastritis.
| 1. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3622] [Article Influence: 120.7] [Reference Citation Analysis (6)] |
| 3. | Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52 Suppl 4:iv7-i11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kapadia CR. Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. J Clin Gastroenterol. 2003;36:S29-36; discussion S61-62. [PubMed] |
| 5. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Yao K, Iwashita A, Yao T. Early gastric cancer: Proposal for a new diagnostic system-based on microvascular architecture as visualised by magnifying endoscopy. Digestive Endoscopy. 2004;16 Suppl:S110-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yao K. Gastric microvascular architecture as visualized by magnifying endoscopy: body and antral mucosa without pathologic change demonstrate two different patterns of microvascular architecture. Gastrointest Endosc. 2004;59:596-597; author reply 597. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nakagawa S, Kato M, Shimizu Y, Nakagawa M, Yamamoto J, Luis PA, Kodaira J, Kawarasaki M, Takeda H, Sugiyama T. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003;58:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Anagnostopoulos GK, Ragunath K, Fortun PJ, Yao K. Identifying Helicobacter pylori-associated gastritis, gastric atrophy and intestinal metaplasia with magnification endoscopy and adaptive index of haemoglobin enhancement technique. Dig Liver Dis. 2005;37:980-981; author reply 982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
S- Editor Wang J E- Editor Liu WF