Published online Jul 28, 2006. doi: 10.3748/wjg.v12.i28.4473
Revised: December 12, 2005
Accepted: January 9, 2006
Published online: July 28, 2006
AIM: To determine the current status in various aspects of gastric cancer patients and to find out the clinical correlation with prognostic role of serum interleukins in Thai patients.
METHODS: Sixty-eight patients were enrolled in this study at King Chulalongkorn Memorial Hospital during April 2003 to May 2005. Gastric cancer was histologically proven in 51 patients and gastric ulcer in 17 patients. Serum IL-6, IL-10, IL-12, and IL-18 levels were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS: There were 26 males (55.32%) and 21 females (44.68%) with their age ranging from 33 to 85 years (mean age 64.49 ± 13.83 years). The common presentations were weight loss (41.2%), dyspepsia (39.2%), and upper gastrointestinal bleeding (15.7%). A total of 35.3% gastric cancer patients and 6.3% of gastric ulcer patients were smokers (P = 0.029). Moreover, 32.4% of gastric cancer patients and 6.3% of gastric ulcer patients were alcoholic drinkers (P = 0.044). Lesion location was pyloric-antrum in 39.4%, gastric body in 39.4%, upper stomach in 12.2% and entire stomach in 6.1% of the patients. H pylori infection was detected in 44.4%. The poorly-differentiated adenocarcinoma was the most common pathologic finding (60.7%). Surgical treatment was performed in 44.1% patients (total gastrectomy in 5.9%, subtotal gastrectomy in 32.4% and palliative bypass surgery in 5.9%). Systemic chemotherapy was given as an adjuvant therapy in 8.8% patients. Carcinomatosis peritoneii were found in 18.8% patients. The mean survival time was 13.03 ± 9.75 mo. The IL-18 level in gastric cancer patient group (58.54 ± 43.96 pg/mL) was significantly higher than that in gastric ulcer patient group (30.84 ± 11.18 pg/mL) (P = 0.0001) (95% CI was 42.20, 13.19). The cut point of IL-18 for diagnosis of gastric cancer was 40 pg/mL, the positive predictive value was 92.31%. The IL-6 level in gastric cancer patients with distant metastasis (20.21 ± 9.37 pg/mL) was significantly higher than that in those with no metastasis (10.13 ± 7.83 pg/mL) (P = 0.037) (95% CI was 19.51, 0.65). The role of IL-10 and IL-12 levels in gastric cancer patients was to provide data with no significant difference.
CONCLUSION: These findings demonstrate that serum IL-6 and IL-18, but not IL-10 and IL-12 levels may be the useful biological markers of clinical correlation and prognostic factor in patients with gastric cancer. Moreover, IL-18 could serve as a diagnostic marker for gastric cancer with a high positive predictive value.
- Citation: Thong-Ngam D, Tangkijvanich P, Lerknimitr R, Mahachai V, Theamboonlers A, Poovorawan Y. Diagnostic role of serum interleukin-18 in gastric cancer patients. World J Gastroenterol 2006; 12(28): 4473-4477
- URL: https://www.wjgnet.com/1007-9327/full/v12/i28/4473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i28.4473
The most recent estimates of the world-wide incidence of cancer indicate that gastric cancer is the second most frequent cancer in the world after lung cancer, with over 900 000 new cases diagnosed every year[1].
For the risk factors, many gastric ulcer patient studies conducted all over the world have shown that the elevated risk is associated with consumption of canned fruit, pickled and smoked foods[2,3]. Many studies have found a positive association between tobacco use and relative risk among heavy drinkers as compared to non-drinkers and gastric carcinoma[4,5]. H pylori, a Gram-negative spiral-shaped bacterium, has been established as a major etiologic agent of chronic gastritis and peptic ulcer diseases including duodenal ulcer (DU) and gastric ulcer (GU). The role of H pylori infection in gastric adenocarcinoma and MALT is also increasingly recognized[6,7].
The metastatic process of gastric cancer consists of tumor cell detachment, local invasion, motility, angiogenesis, vessel invasion, survival in the circulation, adhesion to endothelial cells, extravasation, and regrowth in different organs. In each step, causative molecules have been identified including cell-adhesion molecules, various growth factors, matrix degradation enzymes, and motility factors. Most of these molecules can be regarded as prognostic factors[8].
Human IL-6 consists of 212 amino acids, including a hydrophobic signal sequence of 28 amino acids. Many different types of lymphoid and non-lymphoid cells produce IL-6, which is involved in the following multiple biological activities. IL-6 has a strong activity in stimulating the growth of human gastric cancer cell lines. These findings suggest that IL-6 may play a potential role in the pathogenesis of gastric cancer[9].
IL-10 is an 18 Ku peptide and comprises 178 amino acids. This cytokine was first identified as an anti-inflammatory cytokine. The function of IL-10 is to act on the macrophages, inhibiting synthesis and suppressing gene of other cytokines[10].
IL-12 was originally identified as a natural killer (NK) cell stimulatory factor, a disulfide-linked heterodimeric cytokine composed of 35 and 40 Ku subunits. IL-12, secreted principally by antigen presenting cells (APC), such as macrophages, B cells, and dendritic cells, activates NK cells and T cells to produce interferon-γ (IFN-γ), and augments their cytotoxic activity and proliferation. IL-12 has been recently found to induce anti-tumor effects against a variety of tumors in vivo[11].
IL-18, formerly called interferon-γ-inducing factor, is a recently discovered cytokine that plays an important role in the TH1 response. IL-18 is produced by Kupffer cells, activated macrophages, keratinocytes, intestinal epithelial cells, and osteoblasts. Numerous investigations have noted the importance of IL-18 as a TH1 cytokine, especially in cooperation with IL-12, in anti-tumor immunity[12,13].
Therefore, the purpose of this study was to investigate the possible involvement of serum IL-6, IL-10, IL-12, and IL-18 levels in determining clinicopathologic features and diagnostic yield as well as in predicting the spread of tumors, and most notably including the outcome measured as survival duration for patients with gastric carcinoma.
There were 68 patients enrolled in this study at King Chulalongkorn Memorial Hospital (Bangkok, Thailand) during April 2003 to May 2005. Gastric cancer was histologically proven in 51 patients and 17 gastric ulcer patients served as control. The Ethical Committee of the Faculty of Medicine, Chulalongkorn University, approved the study. Written informed consent was obtained from all patients. Immediately after blood sampling, serum was obtained by centrifugation at 2000 r/min for 15 min at 4°C and stored at 80°C until later analysis. Serum IL-6, IL-10, IL-12 and IL-18 levels were determined using ELISA kits (Quantikine R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions. Briefly, serum samples were reacted with a monoclonal antibody that recognized an epitope of rat interleukins. After 2 h incubation and washing, streptavidin peroxidase-conjugated monoclonal antibody directed to a second epitope was added. This antibody bound to the interleukins captured by the first monoclonal antibody. The color reaction was terminated by a stop solution containing sulfuric acid, and absorbance was measured at 450 nm. Results were calculated from a standard curve generated by a four-parameter logistic curve-fit and expressed in pg/mL.
The clinical data collected included age, sex, symptoms, presentation, history of cigarette smoking and alcohol drinking, lesion site, histological type, metastasis, treatment, H pylori status, blood chemistry, result of treatment, and survival time. The histological diagnosis was based on the morphological examination by hematoxylin and eosin-staining.
Data were expressed as mean ± SD. Comparisons between groups were analyzed by the chi-square test for categorical variables and Student’s t test with 95% confidence interval when appropriate for quantitative variables. Survival curves were constructed using the Kaplan-Meier method. The Pearson correlations were used. P values below 0.05 for a two-tailed test were considered statistically significant. All statistical analyses were performed using the SPSS software for Windows, version 11.5.
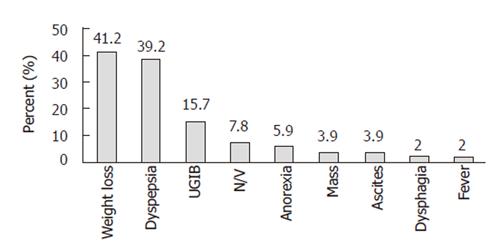
Of the 51 patients with gastric cancer, 26 were males (55.32%) and 21 were females (44.68%) with their age ranging from 33 to 85 years (mean age 64.49 ± 13.83 years). The common presentations included weight loss (41.2%), dyspepsia (39.2%), and upper gastrointestinal bleeding (15.7%) (Figure 1).
A total of 35.3% of gastric cancer patients and 6.3% of controls were smokers (P = 0.029). Moreover, 32.4% of gastric cancer patients and 6.3% of controls were alcoholic drinkers (P = 0.044).
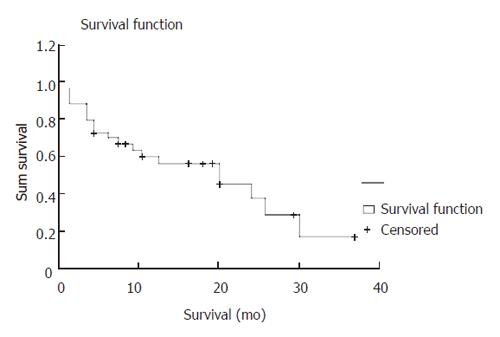
Lesion location was found in pyloric-antrum of 39.4% patients, gastric body of 39.4% patients, upper stomach of 12.2% patients and entire stomach of 6.1% patients. H pylori infection was detected in 44.4% patients. The poorly differentiated adenocarcinoma was the most common pathologic finding (60.7%). Surgical treatment was performed in 44.1% patients (total gastrectomy in 5.9%, subtotal gastrectomy in 32.4% and palliative bypass surgery in 5.9%). Systemic chemotherapy was given as an adjuvant therapy in 8.8% patients. Carcinomatosis peritoneii were found in 18.8% patients. The mean survival time was 13.03 ± 9.75 mo (Figure 2).
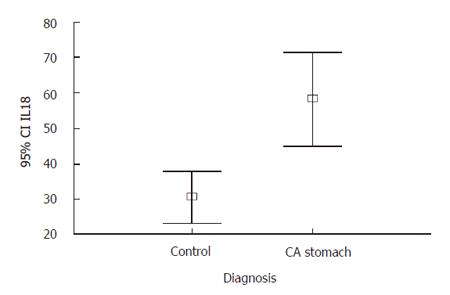
The IL-18 level in gastric cancer patient group (58.54 ± 43.96 pg/mL) was significantly higher than that in control group (30.84 ± 11.18 pg/mL) (P = 0.0001) (95% CI was 42.20, 13.19) (Figure 3). From the ROC curve, the cut point of IL-18 for diagnosis of gastric cancer was 40 pg/mL. The sensitivity was 52.17%, the specificity was 83.33%, and the positive predictive value was 92.31%.
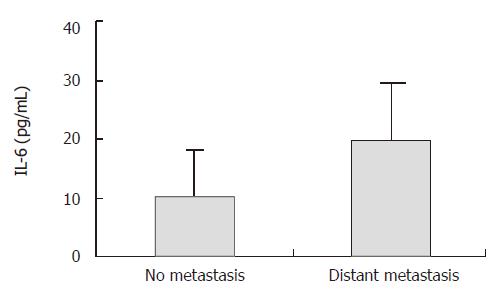
The IL-6 level in gastric cancer patients with distant metastasis (20.21 ± 9.37 pg/mL) was significantly higher than that in those with no metastasis (10.13 ± 7.83 pg/mL) (P = 0.037) (95% CI was 19.51, 0.65) (Figure 4).
A significant clinical correlation was found between hematocrit level, serum albumin, survival time, and serum IL-6, IL-10 and IL-18 levels in gastric cancer patients (Table 1). There was no significant correlation between H pylori status and interleukin levels.
| Correlations | Age | Hct | Alb | Survival(mo) | IL-6 | IL-10 | IL-12 | IL-18 |
| Age | 1.000 | 0.124 | -0.104 | 0.266 | 0.340 | -0.135 | 0.122 | 0.109 |
| Hct | 0.124 | 1.000 | 0.5932 | 0.3371 | -0.4662 | -0.096 | 0.163 | -0.4812 |
| Alb | -0.104 | 0.5932 | 1.000 | 0.4731 | -0.7942 | -0.5002 | -1.0002 | -0.6552 |
| Survival (mo) 0.266 | 0.3371 | 0.4731 | 1.000 | -0.161 | -0.011 | 0.125 | -0.133 | |
| IL-6 | 0.034 | -0.4662 | -0.7942 | -0.161 | 1.000 | 0.182 | -0.243 | 0.2621 |
| IL-10 | -0.135 | -0.096 | -0.5002 | -0.011 | 0.182 | 1.000 | -0.598 | 0.200 |
| IL-12 | 0.122 | 0.163 | -1.0002 | 0.125 | -0.243 | -0.598 | 1.000 | -0.814 |
| IL-18 | 0.109 | -0.4812 | -0.6552 | -0.133 | 0.2621 | 0.200 | -0.814 | 1.000 |
Gastric carcinoma is one of the most common malignant diseases worldwide. Although its incidence has declined dramatically in the United States of America and Western Europe over the past 60 years, the incidence still remains very high in developing countries[14]. The factors leading to this variability among countries are still not clear. Some correlation exists between the occurrence of gastric carcinoma and the prevalence of H pylori infection in different geographical areas[15]. Current knowledge of the detailed mechanisms underlying the interplay between biological modulators and lesions induced by H pylori is still incomplete. It is believed that chronic infection with H pylori leads to alterations of the cell cycle, including increased epithelial cell replication, increased rate of cell death (apoptosis) and production of oxidants[16,17]. In this study, the prevalence of H pylori was 44.4%. There was no significant correlation between H pylori status and interleukin levels.
In agreement with other studies, our study examined the effect of cigarette smoking and alcohol drinking as a risk factor for gastric cancer. From the mechanism viewpoint, the direct carcinogenic effect of cigarette smoking may derive from precursor gastric lesions. Indirect effects of inhaled cigarette smoking may involve the nitrosamines in stomach. Alcohol could act as a contributory factor by causing chronic irritation of the gastric mucosa[4,5].
Weight loss and dyspepsia were the most frequent initial symptoms in our patients, being consistent with those in Western studies[18,19]. The current data indicate that the proportion of early, curable gastric cancer is small among Thai patients. Most of the cases in our study were in advanced stage (stages III and IV) with a very poor 5-year survival.
IL-18 is synthesized as an inactive precursor (pro-IL-18, 24 Ku), which is cleaved by IL-1β-converting enzyme (ICE or caspase-1) into an active 18 kDa mature form[20,21]. IL-18 has multiple biological activities via its capacity of stimulating innate immunity and both Th1 and Th2-mediated responses. It also exerts anti-tumor effects that are mediated by enhancement of NK cell activity, reduction of tumorigenesis, induction of apoptosis and inhibition of angiogenesis in tumor cells[22,23]. In addition, recent data suggest that an inappropriate production of IL-18 contributes to the pathogenesis of cancers and may influence the clinical outcome of patients[24]. This is the first study demonstrating that the levels of serum IL-18 are significantly elevated in gastric cancer patients compared with gastric ulcer patients. Although a highserum IL-18 level was not used as a significant prognostic factor in terms of overall survival, it was used as a diagnostic factor. The cut point of IL-18 for diagnosis of gastric cancer was 40 pg/mL. The sensitivity was 52.17%, the specificity was 83.33%, the positive predictive value was 92.31%. The pathways for IL-18 production and its mechanisms of action in patients with gastric cancer remain to be determined.
IL-6 is involved in many biological activities, including T-cell and B-cell growth and differentiation, IL-2 production and IL-2 receptor expression, hematopoietic stem cell growth, megakaryocyte maturation, acute phase protein synthesis, macrophage differentiation, mesangial cell, keratinocyte, and osteoclast cell growth, hybridoma, plasmacytoma, and myeloma growth, and stimulation of cancer-cell growth[25]. Our data show that the IL-6 level in gastric cancer patients with distant metastasis is significantly higher than that in those with no metastasis. As a proof of the mechanism of IL-6 action, Tamm et al[26] showed that IL-6 makes cancer cells increase their motogenic activity by autocrine pathway. IL-6 secreted from the cancer cells combines with IL-6, which is expressed on the surface of cancer cells, IL-6 and IL-6r act on the cancer cells directly. IL-6 may act through HGF on cancer cells by promoting and accelerating invasion as well as lymph node and/or hepatic metastasis[27].
IL-12 is an immunoregulatory cytokine that triggers the development of a specific T cell-mediated immune response. IL-12 enhances the proliferation, cytokine production, and cytotoxic activity of T lymphocytes and NK cells, with consequent anti-tumor activity[11]. Lissoni et al[28] reported that serum IL-12 level is significantly higher in patients with metastatic renal cell carcinoma and breast cancer than in those with local solid neoplasms. Uno et al[29] reported that phytohemagglutinin-induced production of IL-12 is lower in patients with gastric cancer than in healthy controls, by examining peripheral blood mononuclear cells in vitro. In this study, we could not demonstrate any significant uses of IL-12 in gastric cancer patients as compared with gastric ulcer patients. Shibata et al[30] reported that the production of IL-12 decreases significantly with advancing stages, and is the lowest in patients with distant metastases and cachexia.
For IL-10 level, the data indicate that intracellular IL-10 status on monocytes in patients with advanced gastric cancer is significantly increased compared with those with early disease or healthy individuals[31]. In this study, we also found a correlation between IL6, IL-10, and IL-18 levels and serum albumin. In our previous report, serum albumin level is another prognostic factor in gastric cancer at initial diagnosis, and the median survival time is reduced with a decrease in serum albumin level[32].
In conclusion, our data demonstrate that serum IL-6 and IL-18, but not IL-10 and IL-12 levels, may be the useful biological markers for clinical correlation and prognostic factor in patients with gastric cancer. Moreover, IL-18 could serve as a diagnostic marker for gastric cancer with a high positive predictive value. Thus, the detailed mechanisms of IL-6 and IL-18 involving tumor progression should be further investigated.
The authors thank the Center of Excellence, Viral Hepatitis Research Unit, Chulalongkorn University, Bangkok, Thailand.
| 1. | Pisani P, Parkin DM, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 371] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Gonzalez CA, Sanz JM, Marcos G, Pita S, Brullet E, Saigi E, Badia A, Riboli E. Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer. 1991;49:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Ramon JM, Serra L, Cerdo C, Oromi J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71:1731-1735. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Kune GA, Vitetta L. Smoking and tobacco as an aetiologic factor in gastric carcinoma. GI Cancer. 1995;1:33-38. |
| 5. | Franceschi S, La Vecchia C. Alcohol and the risk of cancers of the stomach and colon-rectum. Dig Dis. 1994;12:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Muñoz N. Gastric cancer and Helicobacter pylori. Eur J Cancer Prev. 1996;5:405-408. [PubMed] |
| 7. | Muñoz N. Is Helicobacter pylori a cause of gastric cancer An appraisal of the seroepidemiological evidence. Cancer Epidemiol Biomarkers Prev. 1994;3:445-451. [PubMed] |
| 8. | Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Ito R, Yasui W, Kuniyasu H, Yokozaki H, Tahara E. Expression of interleukin-6 and its effect on the cell growth of gastric carcinoma cell lines. Jpn J Cancer Res. 1997;88:953-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997;40:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 964] [Article Influence: 34.4] [Reference Citation Analysis (1)] |
| 12. | Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2084] [Article Influence: 67.2] [Reference Citation Analysis (1)] |
| 13. | Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Mühl H, Yoon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658-664. [PubMed] |
| 14. | Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996;23:281-291. [PubMed] |
| 15. | Delchier JC, Ebert M, Malfertheiner P. Helicobacter pylori in gastric lymphoma and carcinoma. Curr Opin Gastroenterol. 1998;14:S41-45. [DOI] [Full Text] |
| 16. | O'Connor F, Buckley M, O'Morain C. Helicobacter pylori: the cancer link. J R Soc Med. 1996;89:674-678. [PubMed] |
| 17. | Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Meyers WC, Damiano RJ Jr, Rotolo FS, Postlethwait RW. Adenocarcinoma of the stomach. Changing patterns over the last 4 decades. Ann Surg. 1987;205:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 454] [Article Influence: 13.8] [Reference Citation Analysis (7)] |
| 20. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1031] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 21. | Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 373] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 22. | Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 311] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Tanaka F, Hashimoto W, Okamura H, Robbins PD, Lotze MT, Tahara H. Rapid generation of potent and tumor-specific cytotoxic T lymphocytes by interleukin 18 using dendritic cells and natural killer cells. Cancer Res. 2000;60:4838-4844. [PubMed] |
| 24. | Lebel-Binay S, Berger A, Zinzindohoué F, Cugnenc P, Thiounn N, Fridman WH, Pagès F. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11:15-26. [PubMed] |
| 25. | Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 984] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 26. | Tamm I, Cardinale I, Sehgal PB. Interleukin-6 and 12-O-tetradecanoyl phorbol-13-acetate act synergistically in inducing cell-cell separation and migration of human breast carcinoma cells. Cytokine. 1991;3:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Lissoni P, Mengo S, Mandalà M, Mauri E, Brivio F, Rovelli F, Confalonieri G, Longarini R, Bonfante A, Folli D. Physiopathology of IL-12 in human solid neoplasms: blood levels of IL-12 in early or advanced cancer patients, and their variations with surgery and immunotherapy. J Biol Regul Homeost Agents. 1998;12:38-41. [PubMed] |
| 29. | Ohno R, Yamaguchi Y, Toge T, Kinouchi T, Kotake T, Shibata M, Kiyohara Y, Ikeda S, Fukui I, Gohchi A. A dose-escalation and pharmacokinetic study of subcutaneously administered recombinant human interleukin 12 and its biological effects in Japanese patients with advanced malignancies. Clin Cancer Res. 2000;6:2661-2669. [PubMed] |
| 30. | Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, Fukuzawa M. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Sugai H, Kono K, Takahashi A, Ichihara F, Kawaida H, Fujii H, Matsumoto Y. Characteristic alteration of monocytes with increased intracellular IL-10 and IL-12 in patients with advanced-stage gastric cancer. J Surg Res. 2004;116:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Thong-Ngam D, Tangkijvanich P, Mahachai V, Kullavanijaya P. Current status of gastric cancer in Thai patients. J Med Assoc Thai. 2001;84:475-482. [PubMed] |
S- Editor Wang J L- Editor Wang XL E- Editor Ma N