Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4431
Revised: September 28, 2005
Accepted: January 9, 2006
Published online: July 21, 2006
To report an extended multivisceral transplantation (MVTx) including right kidney and ascending colon in a patient with complicated Crohn's disease (CD). A 36-year old female suffering from short bowel syndrome and frozen abdomen due to fistulizing CD after multiple abdominal operations underwent MVTx of eight organs including stomach, pancreatoduodenal complex, liver, intestine, ascending colon, right kidney, right adrenal gland, and greater omentum in November 2003. Immunosuppression consisted of alemtuzumab, tacrolimus and steroids. The patient was off parenteral nutrition by postoperative wk 3. She experienced one episode of pneumonia. The patient recovered completely and discharged 2.5 mo and was doing well 30 mo after MVTx. This is one of the very rare cases in which a complete mulitivisceral graft of eight abdominal organs was transplanted orthotopically.
- Citation: Pascher A, Klupp J, Kohler S, Langrehr JM, Neuhaus P. Transplantation of an eight-organ multivisceral graft in a patient with frozen abdomen after complicated Crohn’s disease. World J Gastroenterol 2006; 12(27): 4431-4434
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4431.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4431
Multivisceral transplantation (MVTx) has been attempted since the idea of small bowel transplantation became a clinical reality. Starzl and Kraupp performed the first experimental MVTx in 1960[1]. Starzl was also the first to perform a MVTx clinically in 1983 and 1986[2]. Williams contributed another two MVTx patients whom he reported on in 1989[3]. However, success rates were disappointing with two patients dying early after surgery due to uncontrollable bleeding and the other two due to lymphoma 3 and 6 mo after transplantation. Margreiter et al[4] performed the first MVTx in an adult patient who left hospital free of total parenteral nutrition (TPN). Along with the slow but steady development of intestinal transplantation (ITx) since the late 1980s, the number of MVTx increased as well. Ninety-six MVTx had been reported to the Intestinal Transplant Registry before September 2001, accounting for approximately 13.6% of all intestinal transplants[5]. Because the term MVTx is defined as transplantation of three or more visceral organs en-bloc, there is a considerable variation in number and combination of organs grafted. Additionally, different transplant techniques have been described reflecting the necessity for a tailor-made approach for each patient. Simultaneous MVTx and kidney transplantation (Ktx) have been reported episodically[6-8], however none of them was performed in combination with parts of the colon.
In the following we report a MVTx including sto-mach, pancreatoduodenal complex, liver, intestine, ascending colon, right kidney, right adrenal gland, and greater omentum for a patient suffering from short bowel syndrome and frozen abdomen due to fistulizing Crohn's disease (CD) after multiple abdominal operations. The course was complicated due to secondary end-stage liver disease, decompensated renal insufficiency and sclerosing pancreatitis. This is one of the rare cases in which a complete cluster of eight abdominal organs was transplanted orthotopically. Special focus was put on the technical approach incorporating the right kidney including the right adrenal gland in a straight-forward fashion en-bloc into the multivisceral graft.
A 36-year old female patient was referred to our centre in June 2003 after continuous hospital stay over more than 1.5 years to undergo combined liver and intestinal transplantation because of very short bowel syndrome (15 cm residual jejunum) and total parenteral nutrition (TPN)-associated liver cirrhosis. Fistulizing CD was diagnosed in 1988. In November 2001 subtotal colectomy was performed which was followed by postoperative ileus, recurrent intestinal leakages, recurrent anastomotic leakages with abdominal sepsis and multiple abdominal reoperations resulting in sequential subtotal small bowel resection. TPN was instituted in April 2002 rapidly leading to cholestatic liver disease. TPN-associated progressive liver cirrhosis with severe portal hypertension was diagnosed in autumn 2002. After a total of over 20 operations, the patient suffered from dehiscence of the abdomenal wall until April 2004, when she was referred to our institution. Persistent intra-abdominal and transrectal bleeding due to intra-abdominal abscesses and portal hypertension required 8-10 units of packed red blood cells (RBC) per week. Urgent reoperation revealed extensive intra-abdominal scaring in terms of a frozen abdomen with complete obstruction of the abdominal cavity by the residual mesentery, extensive scaring of the retroperitoneum and an approximately 10 cm dehiscence of the abdominal wall. Hematomas and abscesses were drained, the bleeding situation could be stabilized and a closure of the abdominal wall was performed. However, since a surgical separation, also of the upper abdominal organs, was not possible due to extensive scaring, the indication for en-bloc MVTx was confirmed. In the following months, the patient suffered from recurrent decompensations of liver and kidney function.
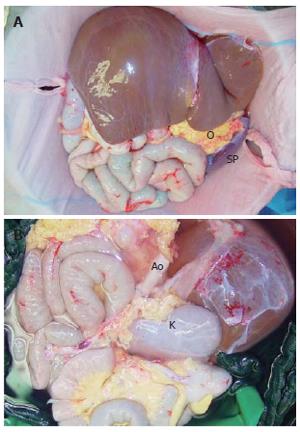
After 5 mo, a blood group and body-weight matched, 13-year old donor was available, who died of brain death four days after a car accident. During donor procedure, preparation was limited to the resection of colon transversum, while the omentum was preserved at the greater curvature of stomach. Later on it was harvested en bloc together with the other grafts. Organs were perfused en bloc with 5 liters UW solution through the aorta, while small bowel perfusion was reduced after 1000 mL by occlusion of the superior mesenteric artery to avoid intestinal edema. The abdominal graft was harvested en bloc, including whole abdominal aorta and caval vein (CV) and all abdominal organs (Figure 1). In the back-table procedure the spleen was dissected and the left kidney was preserved for a different recipient. The aorta was sutured below the origin of the right renal artery in order to build a new celiac trunk as an offspring where all visceral arteries origin.
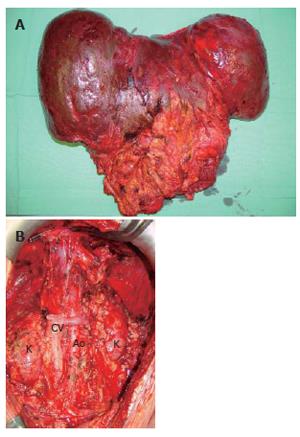
The recipient operation was started with a meticulous surgical dissection and exenteration of the abdominal cavity (Figure 2). The whole abdominal cavity and retro-peritoneum were filled with massive collaterals because of severe portal hypertension which also explained the daily parastomal bleeding episodes prior to MVTx. Although a veno-venous bypass was used between left femoral vein and left axillar vein to reduce the venous blood flow in the abdomen, a total of 71 packed RBCs, 69 units of FFPs, and 5 units of platelet concentrates were transfused during the 11 h operation.
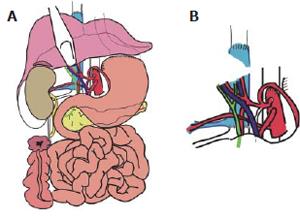
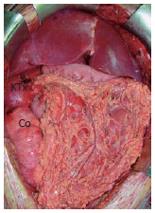
MVTx included stomach, pancreatoduodenal complex, small bowel, ascending colon, liver, right kidney, the adjacent right adrenal gland, and the greater omentum. Following vascular anastomoses were performed: Venous reconstruction was accomplished by inserting the retrohepatic caval vein including the right renal vein (RV) into the recipient (CV) using standard end-to-end anastomosis of the suprahepatic inferior CV and the infrahepatic CV between the insertion of the right donor RV and recipient RV. Arterial reconstruction was performed as previously described[4,7,8] using the donor abdominal aorta with the natural offsprings of the celiac trunc (CT), superior mesenteric artery (SMA), and right renal artery (RA) and inserting it as neo-celiac trunk at the site of the original recipient celiac trunk (Figures 3A and 3B). After a cold ischemia time of 436 min and warm ischemia time of 31 min, reperfusion of the grafts was performed without any complications. Initial function of the organs was excellent including urine production of the transplanted kidney. Reestablishment of intestinal continuity was accomplished by end-to-side gastrostomy of the recipient cardia and donor fundus (Figure 3A), and terminal colostomy of the ascending colon in the lower right quadrant. The right donor ureter was inserted into the right recipient ureter in a side-to-end fashion. The described procedure enabled us to place the transplanted kidney and adrenal gland in almost-orthotopic position just ventrally to the remnant right recipient kidney. Because of the concomitant transplantation of the greater omentum, direct contact of the mesh with the transplanted intestine was avoided (Figure 4). The abdomen could be closed temporarily by an alloplastic mesh and approximated completely within 7 d after transplantation.
Immunosuppression consisted of alemtuzumab (Campath 1H), tacrolimus and steroids. Early enteral immunonutrition was commenced on postoperative d 1. The patient was off TPN by postoperative wk 3. She experienced one episode of pneumonia caused by citrobacter and enterococcus species which was successfully treated. The patient was discharged 2.5 mo after MVTx, with excellent graft function of all transplanted organs. During follow-up she experienced aspergillosis in the left sphenoidal sinus which was treated by drainage and antifungal therapy. There were no further complications and no rejection episodes. Tacrolimus trough levels were adjusted to approximately 7-8 ng/mL 2 mo after MVTx was accompanied by administration of 5 mg prednisolone daily. She was doing well and recovered completely 30 mo after transplantation.
MVTx with varying combinations of visceral organs has been performed increasingly along with the improvements achieved in intestinal transplantation. Combined MVTx and KTx has been mentioned before[9], but only documented in two patients, of them one underwent MVTx without the liver[6,7]. Because recipients of MVTx normally have an extensive history of abdominal surgeries, abdominal exenteration and the transplant procedure itself are technically demanded. Secondary problems such as injury to adrenal glands with the potential effect of adrenal gland insufficiency may arise. We have described here a patient with previous extensive abdominal surgeries after fistulizing CD, subtotal colectomy, recurrent anastomotic leakages and prolonged intra-abdominal sepsis. Since extensive intra-abdominal and retroperitoneal scaring in terms of a frozen abdomen and retroperitoneal sclerosis were present and documented at an earlier point of time before transplantation during a massive intra-abdominal bleeding episode which required emergency surgery, the adrenal gland adjacent to the right kidney was included into the graft. This cautionary measure was proved to be important because neither of the adrenal glands was identifiable due to extensive scaring in the retroperitoneal suprarenal regions and intraoperative bleeding.
Regarding the vascular reanastomosis, two modifi-cations of the originally described[10] and frequently modified procedure for MVTx[11] were used, representing the most appropriate and elegant solution for this patient. The donor abdominal aorta including CT, SMA and right RA was inserted into the recipient aorta as a neo-celiac trunk. Venous reconstruction was conducted in a way that allowed placing the right kidney and adrenal gland in almost orthotopical position with the end-to-end anastomosis between infrahepatic caval veins of the donor and recipient being located just between the right donor and recipient RAs. The piggy back procedure was not used as we were worried about potential venous congestion of the grafted organs when draining into the infrahepatic CV of the donor.
Being aware of recent reports about abdominal wall transplantation for patients with dehiscent abdominal wall after repeat surgery[12], we considered this option in the described patient, too. However, we decided to keep the greater omentum with the graft, thus preserving its natural role in protecting the bowels. Additionally, it induced rapid formation of granulation tissue covering the alloplastic mesh rapidly and leading to rapid reepithelialization of the abdominal wound.
In conclusion, orthotopic MVTx including the right kidney and adrenal gland is technically feasible using end-to-end cavocaval anastomoses. Transplantation of the greater omentum may obviate the need of composite grafts for reconstruction of the abdominal wall in some cases and simultaneous transplantation of the adrenal gland is effective in avoiding adrenal insufficiency due to repeat and extensive surgeries.
| 1. | Starzl TE, Kaupp HA Jr, Brock DR, Butz GW Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Starzl TE, Rowe MI, Todo S, Jaffe R, Tzakis A, Hoffman AL, Esquivel C, Porter KA, Venkataramanan R, Makowka L. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 163] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Williams JW, Sankary HN, Foster PF, Loew JM, Goldman GM. Splanchnic transplantation. An approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA. 1989;261:1458-1462. [PubMed] [DOI] [Full Text] |
| 4. | Margreiter R, Königsrainer A, Schmid T, Koller J, Kornberger R, Oberhuber G, Furtwängler W. Successful multivisceral transplantation. Transplant Proc. 1992;24:1226-1227. [PubMed] |
| 5. | Available from: http://www.intestinaltrans-plant.org/. Access: 05/ 2006. |
| 6. | Rogers J, Bueno J, Shapiro R, Scantlebury V, Mazariegos G, Fung J, Reyes J. Results of simultaneous and sequential pediatric liver and kidney transplantation. Transplantation. 2001;72:1666-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kato T, Nishida S, Levi D, Madariaga J, Nery J, Tzakis A. Multivisceral transplantation without the liver. Transplant Proc. 2002;34:910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Reyes J, Bueno J, Kocoshis S, Green M, Abu-Elmagd K, Furukawa H, Barksdale EM, Strom S, Fung JJ, Todo S. Current status of intestinal transplantation in children. J Pediatr Surg. 1998;33:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Margreiter R. Technical approaches to multivisceral transplantation. Transplant Proc. 2001;33:1543-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Starzl TE, Todo S, Tzakis A, Murase N. Multivisceral and intestinal transplantation. Transplant Proc. 1992;24:1217-1223. [PubMed] |
| 11. | Kato T, Ruiz P, Thompson JF, Eskind LB, Weppler D, Khan FA, Pinna AD, Nery JR, Tzakis AG. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Levi DM, Tzakis AG, Kato T, Madariaga J, Mittal NK, Nery J, Nishida S, Ruiz P. Transplantation of the abdominal wall. Lancet. 2003;361:2173-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF