Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4364
Revised: April 20, 2006
Accepted: April 24, 2006
Published online: July 21, 2006
AIM: To investigate the dynamic change and role of neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) in neonatal rat with intestinal injury and to define whether necrotizing enterocolitis (NEC) is associated with the levels of nitric oxide synthase (NOS) in the mucosa of the affected intestine tissue.
METHODS: Wistar rats less than 24 h in age received an intraperitoneal injection with 5 mg/kg lipopolysaccharide (LPS). Ileum tissues were collected at 1, 3, 6, 12 and 24 h following LPS challenge for histological evaluation of NEC and for measurements of nNOS and iNOS. The correlation between the degree of intestinal injury and levels of NOS was determined.
RESULTS: The LPS-injected pups showed a significant increase in injury scores versus the control. The expression of nNOS protein and mRNA was diminished after LPS injection. There was a negative significant correlation between the nNOS protein and the grade of median intestinal injury within 24 h. The expression of iNOS protein and mRNA was significantly increased in the peak of intestinal injury.
CONCLUSION: nNOS and iNOS play different roles in LPS-induced intestinal injury. Caution should be exerted concerning potential therapeutic uses of NOS inhibitors in NEC.
- Citation: Lu H, Zhu B, Xue XD. Role of neuronal nitric oxide synthase and inducible nitric oxide synthase in intestinal injury in neonatal rats. World J Gastroenterol 2006; 12(27): 4364-4368
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4364.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4364
Necrotizing enterocolitis (NEC) is a common and devastating gastrointestinal condition of neonatal infants[1]. In spite of extensive epidemiological, clinical, and basic research, the pathogenesis of NEC remains elusive, and there is no effective preventative treatment for this disease. Several lines of evidence suggest that neonatal risk factors of prematurity, intestinal asphyxia/ischemia, formula feeding and bacterial colonization all contribute to the occurrence of the disease[1-4]. These components probably act in concert to upset an already immature and delicate intestinal mucosal barrier[5].
Nitric oxide (NO) is a weak radical produced from L-arginine via the nitric oxide synthase (NOS) isoforms: the constitutive neuronal NOS (type I, nNOS), the inducible NOS (type II, iNOS), and the endothelial NOS (type III, eNOS). The endogenous NO is important in defending against bowel injury and mortality[3,6]. Previous studies suggest an important role for iNOS in the pathogenesis of NEC. These include the terminal ileum from rats with abnormal histology demonstrated increased iNOS expression[4,7]. Not all studies, however, have found inhibition of NO production to be beneficial in septic animals or animals challenged with endotoxin. For example, Park et al[8] found that mice receiving a lethal dose of endotoxin and L-NMMA died earlier and had increased lung, liver, and kidney tissue damage than mice receiving endotoxin only. A recent study[9] reported that iNOS knockout mice exhibited an aggravated intestinal injury and inflammation with enhanced neutrophil infiltration in response to acetic acid instillation. Qu et al[10] reported that constitutive NOS is the predominant NOS in the intestine and its activity is inversely correlated with the level of tissue injury. But these experiments were not conducted in newborn animals.
Therefore, the purpose of the present study was to investigate the dynamic change and role of nNOS and iNOS in neonatal rat with LPS-induced intestinal injury and to define whether NEC is associated with the levels of NOS in the mucosa of the affected intestine tissue.
Wistar rats less than 24 h in age (mean weight, 6.24 ± 0.81 g) were given an intraperitoneal (IP) injection of 5 mg/kg E coli O55:B5 endotoxin (LPS; Sigma Chemical Co., St. Louis, Mo, USA) or similar volume of saline[11-13]. All pups were killed respectively at 1, 3, 6, 12 and 24 h after receiving LPS IP (n = 8). Control pups (n = 8) were killed at 1 h after saline IP. The pups which died before collection of the specimens were excluded from the study.
All surviving animals were killed via decapitation. The gastrointestinal tract was carefully removed. The small intestine was then divided into two halves: jejunum and ileum. A 3 cm segment of distal ileum 4 cm proximal to the ileocecal valve from each animal was cut, and fixed for histological evaluation of NEC and immunohistochemical analysis. The rest of the ileum was snap frozen at -80°C for measurements of mRNA.
NEC evaluation: The segment of distal ileum was harvested, fixed in 4% paraformadehyde, embedded in paraffin, microtome-sectioned at 5 μm, and counterstained with hematoxylin and eosin for histological evaluation of intestinal injury. Histological changes in the ileum were scored by a blinded evaluator and were assigned a necrotizing enterocolitis (NEC) score on a scale of 0 to 4 as follows: 0 = normal, intact villous epithelium with normal histology; 1 = mild villous edema, with epithelial sloughing confined to the tips of the villi; 2 = mild midvillous necrosis; 3 = moderate midvillous necrosis, with crypts still readily detectable; and 4 = severe necrosis of entire villi with complete absence of epithelial structures[13,14].
Immunohistochemistry: The localization of nNOS and iNOS was performed by the SABC immunohistochemical technique. The paraffin slices were routinely dewaxed and the antigens were retrieved by microwave. Intrinsic peroxidases were inactivated by 3% hydrogen peroxide solution. The slices were blocked at room temperature for 10 min by normal goat serum. Primary antibody (rabbit anti-rat nNOS and iNOS antibody) in 1:200 dilution was added and left overnight at 4°C. The slices were then washed with PBS, and after biotinylated goat anti-rabbit IgG was added they were left at 37°C for 35 min. They were then washed with phosphate-buffered saline (PBS) and avidin-peroxidase conjugates were added and they were then left at 37°C for 35 min. They were then washed with PBS 3 times (5 min each time) and DAB solution was added for staining at room temperature. The reaction time (5 min) was controlled under the microscope. Slices were then routinely washed and gently restained with hematoxylin and sealed. (The above-mentioned antibody and reagent were purchased from Beijing Zhongshan Biotechnology Co., Ltd). For the negative control group, PBS was used instead of rat nNOS and iNOS antibody. The cells with yellow-brown particle deposition in cytoplasm were judged to be positive.
Five clearly dyed slices at each time point were taken randomly. Then 5 random fields for every slice were selected under microscope (× 40), and the windows’ square was fixed. The contents of nNOS and iNOS were semi-quantitatively measured through optical density average by Meta Morph and computer image process software.
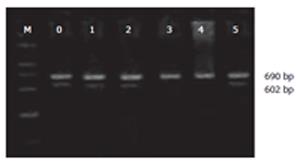
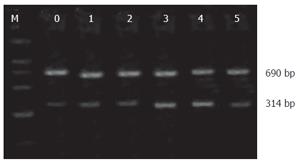
RT-PCR for nNOS, iNOS and β-actin: Total RNA was extracted using the BiotragentsTM reagent (Sino-American Biotechnology Co., Luoyang, China) and 2 μL RNAs were used to synthesize cDNA in the presence of an oligo dT 15-primer, RNase inhibitor and the AMV reverse transcriptase in a final volume of 20 μL. Sequence-specific oligonucleotide primers(Bioasia Biotechnology Co., Ltd, Shanghai, China) were designed according to rat podocin, nNOS, sense: 5’-GAA TAC CAG CCT GAT CCA TGG AA-3’, antisense: 5’-TCC TCC AGG AGG GTG TCC ACC GCA TG-3’; iNOS, sense: 5’-ATC CCG AAA CGC TAC ACT T-3’, antisense: 5’-TCT GGC GAA GAA CAA TCC-3’); β-actin, sense: 5’-CAC CCT GTG CTG CTC ACC GAG GCC-3’, antisense: 5’-CCA CAC AGA TGA CTT GCG CTC AGG-3’. The expected size of amplification was 602 bp for nNOS, 690 bp for iNOS and 314 bp for β-actin. PCR was performed in a 25 μL reaction system which contained 3 μL cDNA, 17.1 μL ddH2O, 10 × PCR buffer 2.5 μL, 2.5 mmol/L dNTPs 2 μL, Taq DNA polymerase 0.2 μL (TaKaRa Biotechnology Co., Ltd, Dalian, China), 0.1 μL of each primer. Amplification cycles of nNOS were 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 1 min and terminated by a final extension of 72°C for 10 min. Amplification cycles of iNOS were 95°C for 1.5 min, followed by 45 cycles at 94°C for 45 s, 55°C for 45 s and 72°C for 1.5 min and terminated by a final extension of 72°C for 10 min.
The PCR products were subjected to electrophoresis with 2% agarose gel and stained with ethidium bromide. The band intensity was determined by gel image analysis system (Kodak 1D, USA). Relative concentrations of mRNA were normalized for β-actin. Expression levels of nNOS mRNA and iNOS mRNA were calculated by dividing the intensity of the internal control, β-actin.
Software SPSS 11.0 for Windows was used in all statistical tests. Comparisons between the groups were calculated using one-way analysis of variance (ANOVA), and all data are expressed as mean ± SD. When P was less than 0.05, the difference was considered statistically significant. The degree of correlation was described using the Spearman’s rank-correlation test.
There was a normal, intact villous epithelium with normal histology of ileum tissue in the control group. Mild villous edema, with epithelial sloughing confined to the tips of the villi could be seen at 1 h following LPS injection, and midvillous necrosis was aggravated from top to the lower part and the amount of the midvillous necrosis in neonate rats was increased with time. The most deteriorating change was at 12 h and the incidence of NEC was 87.5% (7/8). There was severe necrosis of entire villi with complete absence of epithelial structures. The injury score of ileum tissue in the LPS group was also significantly increased than the control group pups (P < 0.01) (Table 1).
The positive expression of nNOS was mainly shown in the nerve plexus and nerve fiber of the small intestinal wall. The myenteric nerve plexus had relatively higher levels of nNOS expression in comparison with the submucous nerve plexus. The positive expression of nNOS was decreased after LPS injection, and was significantly different at 6-24 h following LPS treatment (P < 0.01). The lowest level of its expression was at 12 h (P < 0.001) (Table 2). There was a negative significant correlation between the nNOS and the grade of median intestinal injury within 24 h (γ = -1.000, P < 0.01). The iNOS protein was localized almost exclusively to the epithelial cells (enterocytes) in the surface villi, with some staining detected in the lamina propria. The positive expression of iNOS in LPS group only increased at 12 h (P < 0.05) (Table 2). There was no a significant correlation between the nNOS and the grade of median intestinal injury within 24 h (γ = 0.400, P > 0.05).
Expression of nNOS mRNA in ileual tissue was gradually diminished after LPS injection. The level of its expression was significantly lower at 6 and 12 h (P < 0.05) (Table 3, Figure 1). The expression of iNOS mRNA was significantly increased at 6, 12 h following LPS treatment (P < 0.05) (Table 3, Figure 2).
In the present study, we detected substantially lower levels of nNOS in ileual tissue with complicated intestinal injury when compared with a cohort of control rats. These findings demonstrated a potential role for a deficiency of nNOS in the progression of NEC. There was a significant negative correlation between the levels of nNOS and the degree of intestinal injury. These results showed that decreased levels of nNOS of the ileum result in a dramatic increase of the incidence and severity of NEC. The iNOS mRNA and protein expression was significantly increased during the peak of intestinal injury. The production of NO may lead to consequent enterocyte necrosis, gut barrier failure with further bacterial invasion, and damage to the intestinal wall.
NEC is the most frequent and most lethal disease that affects the gastrointestinal tract of premature infants in neonatal intensive care units, with reported mortality of 10%-30%[15,16]. The reasons for a predilection for prematurity are unclear, but an immature mucosal barrier and immune response likely contribute to the premature neonates’ susceptibility[3,5,17]. Perinatal insults that impair mesenteric circulation may therefore induce intestinal mucosal injury and permit local intestinal microbial flora to breach the mucosal barrier. This process, in turn, initiates an inflammatory cascade leading to NEC[15,18,19].
Over the past decade, an abundance of research has been directed toward the role of NO in intestinal inflammation. NO can exert both beneficial and deleterious effects and that NOS exists in three distinct isoforms: constitutively expressed nNOS and eNOS or iNOS[6]. More than 90% of the total NOS in the small intestine is nNOS. Although iNOS is constitutively present, it accounts for less than 10% of the total NOS activity, and eNOS is barely detectable in the intestine[3,10,20]. iNOS is induced by cytokines or endotoxin and produces large amounts of NO.
The main function of nNOS in the intestine is generally believed to be mediation of the neuronal signal transmission in the NANC components of the nervous system and regulating gut motility. Since eNOS accounts for only a very small part of intestinal NOS activity, it is possible that nNOS in the intestine also functions as the protective eNOS[21].
In the intestine, constitutive NOS may have important physiological functions. The relatively small amounts of NO production by constitutive NOS have been implicated as scavengers of oxidants that may thus protect an organ in the early stages of injury. For example, NO may protect the gastrointestinal mucosa from a variety of stimuli including endotoxin by maintaining mucosal perfusion, inhibiting leukocyte and platelet adhesion to the endothelium, preventing mast cell activation, and acting as an antioxidant[6,22-24]. Our present study showed that the positive expression of nNOS was decreased after LPS injection, which was significantly different at 6-24 h following LPS challenge. The lowest level of its expression was at 12 h. There was a negative significant correlation between the nNOS and the grade of median intestinal injury within 24 h. The expression of nNOS mRNA in the ileum tissue was gradually diminished after LPS injection. LPS may downregulate the expression of nNOS mRNA in damaged ileum and may lead to decreased NO production, which results in impairment of endogenous protective mechanisms in the mucosal barrier[3,6,25]. It may be one of the most important mechanisms resulting in NEC.
The overproduction of NO by iNOS can interact with other reactive oxygen metabolites, such as super-oxide, resulting in the propagation of the highly reactive species, peroxynitrite[26,27]. This potent oxidizing agent can initiate lipid peroxidation and thus produce extreme gastrointestinal mucosal damage and injury[4,9,28]. However, recent studies have challenged this simple paradigm providing evidence that iNOS could also be protective against inflammatory response under certain circumstances[9]. McCafferty et al[29] reported that iNOS deficiency was not important in IL-10 deficient mice with spontaneous chronic intestinal inflammation. Our data showed that the positive expression of iNOS protein only increased at 12 h and the expression of iNOS mRNA was significantly increased at 6, 12 h following LPS injection. The iNOS mRNA and protein expression was significantly increased in the peak of intestinal injury. The production of NO may lead to consequent enterocyte necrosis, gut barrier failure with further bacterial invasion, and damage to the intestinal wall. However, the present study failed to show a clear correlation between the nNOS protein and the grade of median intestinal injury.
In conclusion, the nNOS and iNOS play different roles in neonatal rats with LPS-induced intestinal injury. Caution should be exerted concerning potential therapeutic uses of NOS inhibitors on NEC.
| 1. | Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis. 2003;16:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Di Lorenzo M, Krantis A. Nitric oxide synthase isoenzyme activities in a premature piglet model of necrotizing enterocolitis: effects of nitrergic manipulation. Pediatr Surg Int. 2002;18:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Chan KL, Ho JC, Chan KW, Tam PK. A study of gut immunity to enteral endotoxin in rats of different ages: a possible cause for necrotizing enterocolitis. J Pediatr Surg. 2002;37:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Park JH, Chang SH, Lee KM, Shin SH. Protective effect of nitric oxide in an endotoxin-induced septic shock. Am J Surg. 1996;171:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 10. | Qu XW, Wang H, Rozenfeld RA, Huang W, Hsueh W. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine. Regulation by platelet-activating factor. Biochim Biophys Acta. 1999;1451:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Premer DM, Goertz R, Georgieff MK, Mammel MC, Schwarzenberg SJ. Muscle proteolysis and weight loss in a neonatal rat model of sepsis syndrome. Inflammation. 2002;26:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Qureshi FG, Leaphart C, Cetin S, Li J, Grishin A, Watkins S, Ford HR, Hackam DJ. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Lu H, Li J, Pan LL, Xue XD. Dynamic change of epidermal growth factor in neonatal rat with intestine injury. World J Gastroenterol. 2005;11:3778-3781. [PubMed] |
| 14. | Hammerman C, Goldschmidt D, Caplan MS, Kaplan M, Bromiker R, Eidelman AI, Gartner LM, Hochman A. Protective effect of bilirubin in ischemia-reperfusion injury in the rat intestine. J Pediatr Gastroenterol Nutr. 2002;35:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Bister V, Salmela MT, Heikkilä P, Anttila A, Rintala R, Isaka K, Andersson S, Saarialho-Kere U. Matrilysins-1 and -2 (MMP-7 and -26) and metalloelastase (MMP-12), unlike MMP-19, are up-regulated in necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2005;40:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156-G164. [PubMed] |
| 18. | Yost CC. Neonatal necrotizing enterocolitis: diagnosis, management, and pathogenesis. J Infus Nurs. 2005;28:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 289] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Qu XW, Rozenfeld RA, Huang W, Sun X, Tan XD, Hsueh W. Roles of nitric oxide synthases in platelet-activating factor-induced intestinal necrosis in rats. Crit Care Med. 1999;27:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Qu XW, Wang H, De Plaen IG, Rozenfeld RA, Hsueh W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001;15:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Luo CC, Chen HM, Chiu CH, Lin JN, Chen JC. Effect of N(G)-nitro-L-arginine methyl ester on intestinal permeability following intestinal ischemia-reperfusion injury in a rat model. Biol Neonate. 2001;80:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kitchen PA, Walters JR. Molecular and cellular biology of small-bowel mucosa. Curr Opin Gastroenterol. 2001;17:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Gobert AP, Mersey BD, Cheng Y, Blumberg DR, Newton JC, Wilson KT. Cutting edge: urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J Immunol. 2002;168:6002-6006. [PubMed] |
| 25. | MacKendrick W, Caplan M, Hsueh W. Endogenous nitric oxide protects against platelet-activating factor-induced bowel injury in the rat. Pediatr Res. 1993;34:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Chan KL, Hui CW, Chan KW, Fung PC, Wo JY, Tipoe G, Tam PK. Revisiting ischemia and reperfusion injury as a possible cause of necrotizing enterocolitis: Role of nitric oxide and superoxide dismutase. J Pediatr Surg. 2002;37:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Potoka DA, Nadler EP, Upperman JS, Ford HR. Role of nitric oxide and peroxynitrite in gut barrier failure. World J Surg. 2002;26:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G90-G99. [PubMed] |
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH