Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4281
Revised: April 28, 2006
Accepted: May 25, 2006
Published online: July 21, 2006
The gastrointestinal tract is lined by a simple epithelium that undergoes constant renewal involving cell division, differentiation and cell death. In addition, the epithelial lining separates the hostile processes of digestion and absorption that occur in the intestinal lumen from the aseptic environment of the internal milieu by defensive mechanisms that protect the epithelium from being breached. Central to these defensive processes is the synthesis of heme and its catabolism by heme oxygenase (HO). Dietary heme is also an important source of iron for the body which is taken up intact by the enterocyte. This review describes the recent literature on the diverse properties of heme/HO in the intestine tract. The roles of heme/HO in the regulation of the cell cycle/apoptosis, detoxification of xenobiotics, oxidative stress, inflammation, development of colon cancer, heme-iron absorption and intestinal motility are specifically examined.
- Citation: Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol 2006; 12(27): 4281-4295
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4281
The lumen of the intestine mucosa is predominately covered with epithelial cells called enterocytes which are responsible for the terminal digestion and absorption of nutrients. These cells have a limited lifespan before being replaced by cells derived from the crypt region[1]. There is also evidence of apoptosis within the crypt, presumably in response to excess cellular proliferation, cytotoxicity or genomic damage[2]. Surviving cells undergo apical migration, limited cell replication, commitment and differentiation[1]. The process of differentiation is gradual, characterised by the accumulation of cell-specific products in the upper crypt region and attaining the mature phenotype in the lower to middle-villus region. Recent evidence indicates that heme is important in intestinal development as well as maintaining the mucosal barrier and protecting the body from invasion and the damaging consequences of ingested xenobiotics. However, heme in the colon may irritate the mucosa and derange the normal rates of proliferation/exfoliation, circumstances that raise the probability of colon cancer. Heme is also an important source of body iron and how it is absorbed by the enterocyte is considered in this article, as well as the role heme plays in intestinal motility. It needs to be recognised that an in depth focus on each of these components is outside the scope of this review, rather it is our intention to provide the general reader with evidence and interpretations supporting the markedly varied involvement of heme in intestinal function.
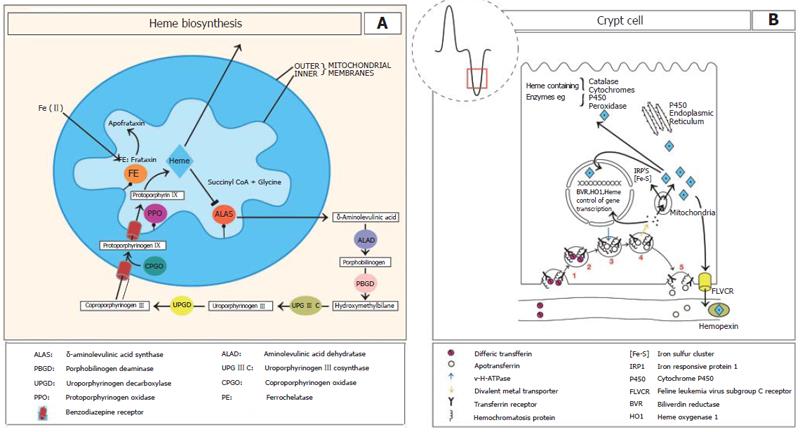
Heme biosynthesis involves 8 enzymes, four localised to the cytoplasm and the others in the mitochondrial matrix[3-5] and is regulated by the first enzyme in its synthesis aminoleuvilinic acid synthase[6] (Figure 1A). Heme biosynthesis also requires iron, which in the intestinal crypt is derived from the plasma by the activity of the transferrin receptor operating in collaboration with the hemochromatosis protein (HFE)[7] (Figure 1B). Although heme synthesis is highest in the crypt epithelium it continues along the length of the crypt-villus axis. As the cells leave the crypt region iron appears to be acquired from the diet since dietary iron deficiency reduces the heme content of villus enterocytes, and in villus cells transferrin receptor has 25% the activity of crypt epithelium[8,9] (Figure 1B).
HO catalyses the mixed function oxidation of heme using cytochrome P-450, NAPDH and molecular oxygen[10-12]. HO functions in the oxidative cleavage of heme specifically at the α-methane bridge, resulting in the formation of biliverdin IXα which is rapidly reduced to bilirubin IXα by soluble biliverdin reductase (BVR). Since tissue BVR activity is 30-50 times greater than HO activity, this suggests that it is unlikely to limit heme breakdown, and that the rate limiting component is HO[12]. Recently, the crystal structure of HO in complex with heme and biliverdin-iron has been solved[13]. HO binds heme and oxygen between two helical folds with the proximal fold binding heme while the distal helix contains an oxygen binding site[13].
HO is expressed as two isoforms designated HO-1[14] and HO-2[14,15] which are products of different genes[14]. HO-1 shares substantial homology with HO-2[15]. The molecular mass of HO-1 is 32 kD, while HO-2 is 36 kD. HO-1 expression is induced by numerous factors, including oxidative stress, inflammation, cytokines, nitric oxide, prostaglandins, an elevated level of substrate[16], iron deficiency[17], metals including Cd, Co, Cr, Cu, Fe, Hg, Ni, Pd, Pt, Sn, Zn[3,16,18,19], hyperoxia[20] and UV light[21]. The induction of HO-1 by hyperthermia has led to use of an alternate name, heat shock protein 32 (HSP-32)[22]. Unlike the inducible expression of HO-1, HO-2 expression is relatively constant.
HO-1 is mainly involved in the reutilization of heme-iron from hemoglobin and the expulsion of iron from tissue stores as evidenced by HO-1 knockout mice which develop anaemia because of progressive tissue iron retention particularly within macrophages[23]. A previous study shows that less than 50% of endogenous hepatic heme degradation in rats is accounted for by HO-1 activity as evidenced by the generation of CO from heme[24]. Therefore there appear two separate fates for catabolized heme-iron. Firstly a HO-1 dependent pathway, where iron from heme passes efficiently from the macrophage to the plasma, probably by the iron transporter ferroportin[25], and secondly, a HO-1 independent pathway which results in retention of the freed iron.
HO-1 functions to diminish cellular oxidative stress because HO-1 reduces the levels of the pro-oxidant heme and produces the antioxidant bilirubin[26]. Supporting this, humans deficient in HO-1[27] and individuals with impaired transcription due to a microsatellite polymorphism in the HO-1 promoter region[28,29] present with a phenotype similar to HO-1 knockout mice[30]. Interestingly, HO-2 is unable to compensate for the loss of HO-1, probably because its expression is restricted to a select group of cells or it is unable to be induced to the levels of activity required to produce the effects seen with HO-1 expression[27-30]. HO-1 and intestinal oxidative stress is discussed in a later section.
The synthesis of heme and heme-containing proteins is crucial for intestinal function. These hemoproteins include electron carrying proteins such as cytochrome (CYP) P450 (see section on detoxification), mitochondrial localised cytochromes, the ferrireductase Dcytb[31], catalase and peroxidases which catalyse the reaction of hydrogen peroxide (H2O2) to water and oxygen (see section on oxidative stress). In addition to biosynthesis, heme can also be acquired by the enterocyte via intestinal absorption. This will be discussed in detail below with respect to the intestine.
In the human intestinal cell lines CaCo-2 and HT-29, internalisation of heme increased HO-1 expression, indicating that the heme responsive element in the promoter region of the HO-1 gene was accessible and functional[32,33]. Duodenal HO-1 expression is also increased in iron deficiency[17] and by conditions that lead to oxidative stress including heavy metals and inflammation (see below with respect to the intestine). Up-regulation of HO-1 gene expression via the estrogen receptor β[34], octreotide, a somatostatin analogue[35] and glutamine[36] has been established. HO-2 expression is constitutive and mainly confined to the enteric nervous system and interstitial cells of Cajal, although it is possible that HO-2 is expressed by enterocytes[37]. This will be addressed later in this review.
Heme turnover is the balance between heme synthesis and its destruction by heme oxygenase. It is subject to variation along the crypt-villus length, being highest in the crypt and least at the villus tip[38]. Thus the crypt region has the highest activity of both heme biosynthesis and heme oxygenase activity. As the cells migrate the rate of heme synthesis decreases but destruction decreases to a lesser extent, therefore total heme content is highest at the villus enterocytes compared with crypt epithelium.
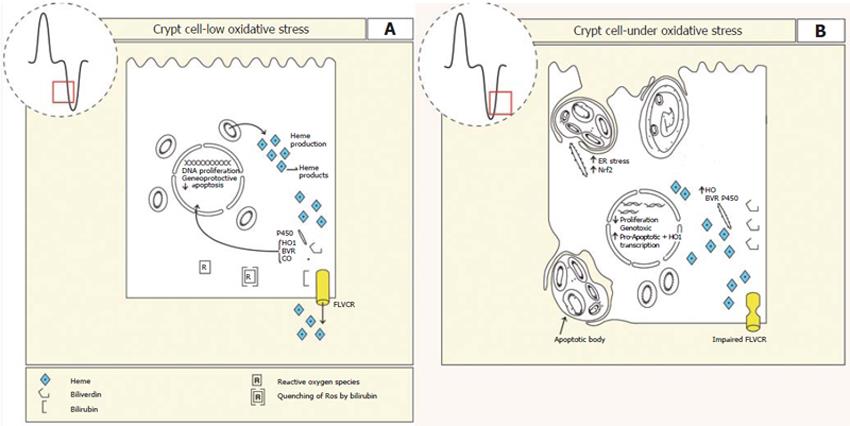
Cell turnover and differentiation is a function of crypt epithelium. Similar to that seen in the crypt epithelium, HO-1 activity is highest in undifferentiated intestinal epithelial Caco-2 cells[39]. This suggests that HO-1 and cell proliferation/apoptosis may be linked[40]. Supporting this, inhibiting HO-1 activity reduced cell proliferation and increased cell death[40,41]. Conversely, in the human intestinal cell line HT-29 cells induction of HO-1 activity reduced expression of the pro-apoptotic gene caspase-3 and inhibited apoptosis. This supports the idea that HO-1 activity is anti-apoptotic[42]. It is possible that HO-1 mediates these effects indirectly on gene transcription via the activity of BVR (Figure 2).
BVR (EC 1.3.1.24) must undergo auto-phosphorylation in order to convert biliverdin to bilirubin[43]. This property of phosphorylation/dephophorylation during the conversion of biliverdin to bilirubin is similar to that seen with signalling kinases. Recent evidence indicates that BVR functions as a serine/threonine kinase that operates in the insulin receptor/MAPK pathways[44] and a transcription factor with a bZip domain involved in ATF-2/CREB and HO-1 regulation[45]. These additional roles suggest that BVR may have a broader function in regulating cellular activity[46]. Since BVR immunoreactivity is seen in nuclei of epithelium lining the GI tract, this suggests a possible role in the regulation of gene transcription[47].
It is possible that HO-1 may modulate proliferation by scavenging and/or preventing the formation of reactive oxygen metabolites (ROM) and reactive nitrogenous metabolites (RNM), since ROM inhibit Caco-2 cell proliferation[48] and stimulate apoptosis[49]. This is particularly relevant to the intestinal crypt region where proliferation exists and the levels of antioxidant detoxifying enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase and catalase are low[50]. If this is true then HO-1 level in the crypt region may act in defence against oxidative stress to limit mutation of DNA. HO-1 may therefore be one guardian of the genome, limiting mutations of DNA and promoting deletion of aberrant cells (Figure 2).
As discussed previously the production of heme for enzymes, electron transport and as substrate for activity of HO1 and BVR is likely to be finely balanced since excess heme leads to oxidative stress and subsequent cell damage. Therefore as differentiation concludes heme production must fall. This may be achieved through reduced heme biosynthesis, increased HO-1 activity or increased heme export. With respect to heme export, a human heme exporter with homology to Feline leukaemia virus, subgroup C receptor (FLVCR) has recently been identified which has a clear function in erythropoiesis at the CFU-E stage of development[51]. Impairment of FLVCR leads to the loss of CFU-E cells and impairs erythroid differentiation by inducing apoptosis. FLVCR is also expressed by Caco-2 cells, suggesting that it may be involved in intestinal differentiation by reducing the cellular heme concentration as the cell differentiates[51]. This would reduce the oxidative burden on the stem/progenitor cell and potentially limit genomic damage[52]. Supporting the existence of the FLVCR in the intestine, Caco-2 cells internalised heme by an active transport process and transcytosed it from apical to basal surfaces[53]. The converse was also true. Exposing the membranes to trypsin selectively increased the rate of uptake across the apical membrane only. Taken together these results raise the possibility that heme can be actively secreted from the cell in either direction possibly involving FLVCR (Figure 2).
HO activity is highest in the duodenum and lowest in the terminal ileum[54-56]. This pattern of HO activity appears to correlate with the uptake of ingested xenobiotics and heme-iron absorption along the length of the intestinal tract (see below). In fact, treating rats with phenobarbital increased microsomal P450 enzyme activity, and absorption of iron from hemoglobin[57]. Conversely, when an inhibitor of intestinal HO activity was given, intestinal heme-iron absorption decreased[58] (see below).
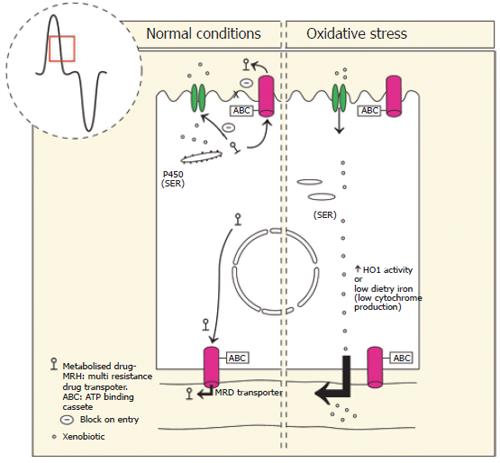
The intestine makes an important contribution to the detoxification of many ingested xenobiotics (food additives, industrial chemicals, pesticides, plant toxins and pharmaceutical agents)[59-61]. The heme containing P450 enzymes in particular the CYP3A superfamily are an integral component of xenobiotic detoxification. P450 levels are highest in the proximal duodenum, falling to lowest levels at the ileum[62,63]. This correlates with the gradient of exposure to ingested xenobiotics. The highest activity of the P450 enzymes studied to date is the villus region[64-67]. Interestingly, ingested xenobiotics induce greater CYP activity in the crypt epithelium compared with villus enterocytes[66]. Since the crypt cells do not absorb nutrients, this suggests that they passively absorb the drug or that the drug is actively absorbed by enterocytes and then taken up from the plasma by crypt cells. This interpretation is consistent with highest levels of heme biosynthesis in crypt epithelium.
Detoxification involves three phases, firstly the CYP450s and its mixed function oxidases adds a reac-tive group to the xenobiotic, secondly the molecule is made water soluble by conjugation to glucuronic acid, sulphates, glutathione or amino acids by UDP-glucuronosltransferases [UGT], sulfotransferases [SULT] or glutathione S-transferases [GST], respectively, thirdly the metabolite is excreted from the enterocyte into the lumen by a transporter such as the ATP binding cassette transporters (ABC), P-glycoprotein[59,62,63]. This “first pass” detoxification of xenobiotics is most active in the upper villus where absorption of nutrients and xenobiotics are greatest[64-67].
To perform optimal detoxification the enterocyte must express appropriate levels of CYP450 and this is in part determined by heme turnover. Therefore for the enterocyte to express appropriate CYP450, adequate absorption of iron from the diet is required for heme synthesis along with conditions that limit HO-1 expression[68-70]. If HO-1 activity is induced, for example by ingestion of environmental contaminants such as cadmium, organotins and heavy metals increased destruction of CYP will take place and first pass detoxification will be compromised. Similarly, iron deficiency reduces the ability to synthesise heme and therefore detoxify xenobiotics[64,65,71]. This may therefore predispose an individual to cancer and ulceration of the colon[72] (Figure 3).
Several metalloporphyrins are competitive inhibitors of HO-1 activity because they have the capacity to interact with the heme binding site in HO-1, but are unable to activate the enzyme. This leads to a loss of heme degradation[73-76]. This strategy has been used in the correction of human neonatal hyperbilirubinemia[77-79]. Treatment with tin-protoporphyrin/mesoporphyrin, two structurally related heme analogues are effective in lowering serum bilirubin levels in many animals by competitively inhibiting HO[73-79]. In addition, the use of short interfering RNAs targeting HO-1 mRNA expression has also been proposed to treat hyperbilirubinemia[80]. Although there is a recognised loss of endogenous heme through the bile during metalloporphyrin administration[81-83] that has been linked to an iron deficient state[84], the iron deficiency has been shown to be readily reversible.
In the enterocyte, bilirubin is conjugated to glucuronic acid by bilirubin glucuronyl-transferase and excreted into the intestinal lumen[85], or passed into the plasma where it non-covalently binds albumin and is transported to the liver, conjugated and excreted into the bile. However, early in perinatal life the luminal activity of secreted lysosomal-derived glucuronidase is high suggesting that enterocyte and biliary excreted conjugated-bilirubin can be deconjugated within the intestinal lumen enabling bilirubin to be reabsorbed via the enterohepatic circulation[86]. This would contribute to neonatal hyperbilirubinemia.
Glutamine is a major source of energy for the enterocyte and has been shown to promote intestinal growth and maintain intestinal integrity particularly when the intestine is heat stressed and starved[36,87-91]. Glutamine stimulates intestinal proliferation and acts synergistically with epidermal growth factor to induce the mitogen-activated protein kinases and Jun nuclear receptor kinases. These in turn phosphorylate nuclear transcription factors such as AP-1 which activate transcription of target genes involved in cell proliferation and repair, including HO-1[36,88]. Recently it was shown that glutamine stimulation of HO-1 expression was protective against endotoxic shock of the lower intestine[90].
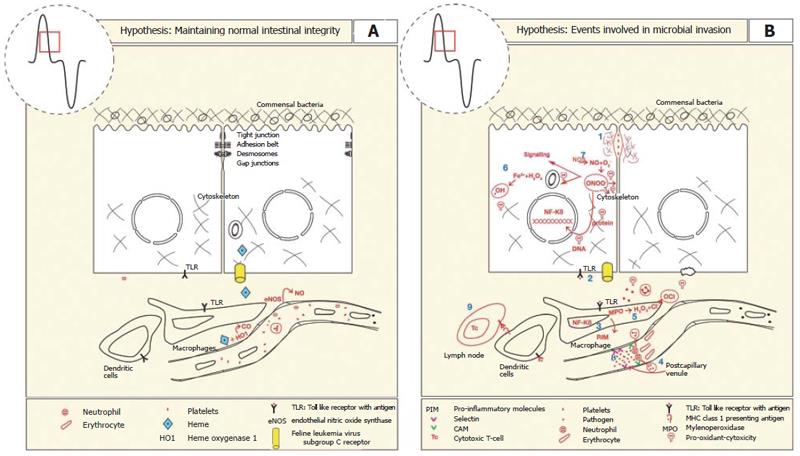
The epithelium lining the gastrointestinal tract presents a “mucosal barrier” to the migration of pathogens into the lamina propria that reside within the lumen of the gastrointestinal tract. In addition to the epithelium which is selectively permeable to nutrient absorption, the mucosal barrier comprises tight junctions that prevent migration of pathogens between cells. Breaching the mucosal barrier elicits an inflammatory response which first involves the innate immune system. Toll like receptors (TLR) expressed on the basolateral surface of enterocytes and the cell membrane of macrophages are activated[92] and these in turn activate intracellular signalling pathways that induce NF-κB dependent transcription of genes involved in the pro-inflammatory response such as cytokines, chemokines, immune receptors, nitric oxide synthase, prostaglandins and cell surface adhesion molecules[93-95]. The pro-inflammatory mediators initially function to increase blood flow and edema. Concomitant with this, endothelial cell membranes express cell adhesion receptors including ICAM-1 that enable white blood cells to adhere and extravasate[34]. The further release of pro-inflammatory chemokines (CINC-1, -3) may lead to hemostasis and organ failure[34].
Inflammation is known to induce HO-1 gene expre-ssion and in turn its activity. The bilirubin and CO produced are thought to have restorative effects on impaired tissue function, in the case of bilirubin it is a potent anti-oxidant[26,96-98]. There was increased oxidized bilirubin in the urine of patients following invasive surgery, supporting the idea that bilirubin acts as an antioxidant to scavenge reactive oxygen species[97].
The second metabolite of HO-1 activity, CO has been shown to relax vascular smooth muscle by binding to the heme moiety of soluble guanyl cyclase (sGC). Activation of sGC increases blood flow to the site of intestinal injury[99,100], inhibits platelet aggregation[101], reduces microvascular fibrin accumulation[102] and restricts leukostasis in postcapillary venules[93,103]. Reduced leukostasis by CO is thought to occur via inhibition of the expression of the adhesion molecules, P-, E- selectins, and ICAM although some contribution by bilirubin is also thought responsible for the leukostasis[104-106]. CO exerted additional cytoprotection by inhibiting components of the pro-inflammatory pathway including TNF-α, IL-1β, IL-2, IL-6, interferon-γ and cyclo-oxygenase, while stimulating the anti-inflammatory cytokine IL-10[42,107-114] (Figure 4).
The third metabolite resulting from HO-1 activity is Fe(II). If this reaches the labile iron pool it will induce oxidative stress by participating in Fenton and Haber Weiss driven reactions and this would exacerbate inflammation. However, this is avoided by sequestration by ferritin[21].
Nitric oxide synthase (NOS) is a heme-containing enzyme that converts L-arginine to nitric oxide (NO) and citrulline. Similar to CO, NO binds the heme moiety of guanylate cyclase to produce vascular smooth muscle relaxation. Under normal circumstances eNOS/NO is important in maintaining mucosal integrity by modulating intestinal blood supply. NO at low concentrations stimulates mucous production, electrolye secretion and decreases pro-inflammatory responses of mast cells, neutrophils, platelets and endothelial cells[115-117] (Figure 4A).
During inflammation cytokines activate NF-κB dependent gene expression of iNOS by intestinal epithelial cells, neutrophils and macrophages. This leads to production of NO[116-118] at considerably higher levels than by eNOS activity. At this concentration NO reacts with superoxide anions to form the cytotoxic reactive nitrogen metabolite, peroxynitrite[119-123]. Although peroxynitrite destroys micro organisms, it also reversibly inhibits heme containing proteins including cytochrome C, catalase, cytochrome P-450 and cytoskeletal proteins[120,122]. It was suggested that inhibition of iNOS during endotoxin-induced gut mucosal dysfunction was beneficial because mitochondrial oxidative metabolism was unimpaired[119]. This leads to maintenance of mucosal barrier integrity that resists bacterial translocation[124] (Figure 4B).
Collectively, these findings indicate that at low concentrations NO maintains mucosal integrity, but at high concentrations NO induces reactive nitrogen metabolites which impair intestinal function.
During intestinal inflammation HO-1 mRNA expression increases in response to the activity of NO[125]. It is likely that this is due to increased transcription and stabilization of existing transcripts[125]. In addition, induction of HO-1 in a human intestinal cell line resulted in the degradation of cytokine-induced NOS. This reduced the production of NO and therefore peroxynitrite[124]. Heme was also shown to reduce the NOS mRNA[124]. The inhibition of NOS activity by HO-1 was lost when tin protoporphyrin was given, indicating the direct effect of HO-1 in regulating NOS activity[126]. These findings are consistent with a role for HO-1 in limiting the deleterious effects of excessive iNOS by directly inhibiting its transcription, degrading existing NOS and scavenging excess ROM/RNM with bilirubin.
In western civilisations, 40% of the average non-vegetarian person’s total body iron is derived from heme products. However, iron from these substances only constitutes 15% of ingested iron[127,128], suggesting that heme-iron is more efficiently absorbed than non-heme iron. This observation also explains why vegetarians are more prone to iron deficiency than meat eaters. Despite the importance of the contribution of heme to body iron stores, how it is absorbed is still poorly understood.
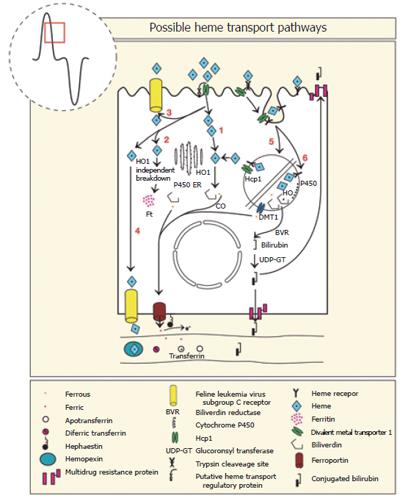
It is generally recognised that in omnivorous animals, heme is not transferred into the blood as an intact metalloporphyrin, instead absorption of iron from heme involves three steps (1) Uptake of luminal metalloporphyrin [Fe(II)-protoporphyrin-IX] by the enterocyte (2) catabolism within the enterocyte, combining of pools of ingested iron from non-heme and heme sources and (3) release of elemental iron to the bloodstream by the enterocyte[129-133]. A large number of proteins are thought to be involved in the mechanism of heme iron absorption and these are tabulated along with their sites of expression and function (Table 1). Most of these proteins will be discussed individually in the following sections and is also summarised in Figure 5.
| Protein | Function | Location | Regulation by Fe |
| Heme receptor | Receptor for heme | ? | Inversely |
| HCP1 | Transporter of heme | AM -> BC | Constant |
| FLVCR | Heme exporter | ? | Unknown |
| Ferritin | Iron storage | C | Directly |
| DMT1 | Fe(II) importer | AM+Lys | Inversely |
| Ferroportin | Fe(II) exporter | BL AM | Inversely |
| Hephaestin | Ferroxidase + ? | SN, BL | Constant |
| HO 1 | Degradation of heme | C | Inversely |
| HO 2 | Degradation of heme | SMC, EN | Constant |
| HFE | Regulator | TW | Inversely |
| TfR1 | Tf:Fe endocytosis | BL, SN | Constant |
| Transferrin | Endosomal iron transport | C | Inverse |
Worthington and co-workers used immunofluorescent methods to show that the uptake of a heme analog was temperature and time dependent, could be inhibited by heme competition and augmented by inhibitors of heme synthesis[134]. It is likely that Worthington and co-workers identified a heme transport process by Caco-2 cells that may be a transporter and/or possibly a heme receptor.
Heme is taken into the enterocyte intact as evidenced by the recovery of 59Fe-heme from the small intestinal mucosa following the gavage of radiolabelled hemoglobin[130-133]. This process is energy dependent indicating an active process[135]. The finding that absorption of iron from hemiglobin and hemoglobin were equivalent suggests that uptake of heme is independent of the redox state of the heme-iron[136,137]. Alternatively there is an oxidoreductive mechanism on the cell surface that is capable of converting the iron redox state before internalization.
A microvillus membrane transporter that imports heme from the lumen into enterocytes of mice was recently characterised[138]. This protein was expressed in the duodenum but not the ileum, consistent with expression at the site of highest heme-iron absorption. Heme carrier protein 1 (HCP1) encodes a protein with strong homology to bacterial tetracycline-resistance proteins, which are characterised as having 12 transmembrane domains and are members of the major facilitator superfamily[138]. Functional characterisation of HCP1 using Xenopus oocytes revealed selectivity for the transport of heme but not tetracycline or non-heme iron. In vitro studies involving HCP1 siRNA and in vivo studies blocking HCP1 activity by antibodies indicated that the uptake of heme fell. HCP1 also required energy but the source of energy is presently unknown. Collectively, these findings indicate the first functional characterisation of a heme specific transporter.
Interestingly, during conditions known to increase non-heme iron absorption such as hypotransferranemia and iron deficiency, HCP1 mRNA expression remained constant although it was increased by hypoxia. Similarly, the extent of HCP1 protein expression remained constant with respect to the iron content of the enterocyte, although the protein translocated from the microvillus membrane to the basal cytoplasm during iron loading. The lack of increased expression of HCP1 by iron deficiency may in part explain the limited ability to increase heme-iron absorption. It may also indicate that HCP1 needs additional modulating proteins in order to regulate heme-iron absorption (Figure 5).
Previous studies have reported a 50% increase in heme binding to microvillus membrane preparations during iron deficiency, raising the possibility of a brush border localised heme receptor[139-142]. This is based on the measurements of binding [14C]-heme to semi-purified brush border preparations[139-142]. Subsequent solubilisation of the brush border microvillus membranes identified the size of the heme binding substances, one with a molecular mass of about 250 kDa the other about 60 kDa. Displacement of the [14C]-heme by unlabelled heme was seen with the 250 kDa complex, but not the about 60 kDa complex[139-141], suggesting the larger peak represented a heme receptor complex, while the smaller peak was thought to be polymerised heme[140]. Based on the capacity of the large complex to be saturated with heme and having an Ka of 10-6 to 10-7 mol/L this suggests that it is a relatively high affinity heme receptor.
In addition to the identification of a putative heme receptor in the intestine, others have identified a heme binding protein that is distinct from the hemopexin receptor[143] with similar binding characteristics to the intestinal heme receptor. Since the heme binding protein and HCP1 have molecular weights of about 250 kDa and about 50 kDa, respectively, it is unlikely they are the same protein, unless HCP1 forms part of a larger complex. The finding that erythroleukaemic cells internalise heme coated latex beads[144,145] and that trypsin treatment eliminates heme binding[146,147] supports the existence of a heme receptor-mediated, endocytotic pathway. It therefore appears that there are at least two defined pathways involved in the uptake of heme into the enterocyte, one involving HCP1[138] and the other a receptor-mediated endocytotic process[139-142,144-147]. Despite considerable characterisation of the heme receptor almost thirty years ago there has been little progress made since (Figure 5).
Morphological studies show that following ingestion of a heme-rich meal by rodents, heme was first seen along the microvillus membrane, then in tubulovesicular structures of the apical cytoplasm and finally in secondary lysosomes[148,149]. Based on time course studies, DAB (3,3-Diaminobenzidine tetrahyhydrochide) disappeared from lysosomes suggesting that heme was either transported from these structures or that it was degraded within them. In either case heme degradation involves HO activity but whether this is HO-1 or HO-2 is presently unknown.
In rats treated with alcohol there was increased absorption of iron from heme as well as the entire hemoglobin complex where it was transported to the liver as a haptogobin-hemoglobin complex[150,151]. Thus, absorption of iron from hemoglobin also appears to contribute to the iron over loading caused by excessive alcohol consumption.
The intracellular sites where restrictions to the absorption of iron from heme occur have been studied in dogs given radiolabelled hemoglobin and then measuring the progression of radioactivity through mucosal compartments[133]. The most likely sites where the rate of iron absorption was limited appears to be at the stage of heme breakdown and/or the release of iron from the cell. This might involve the steps where HO operates, where iron is released out of an intracellular compartment, or from the cell (see below).
In view of the likely convergence of iron derived from sources of non-heme and heme iron what is known for non-heme iron is described.
The Microcytic mouse (mk) and anaemic Belgrade rat (b) have an autosomal recessive inherited, hypochromic, microcytic anaemia associated with a well-characterised defect in the transferrin cycle in erythroid cells[152], as well as a defective intestinal non heme-iron transport that is manifest at the site of uptake at the microvillus membrane[153]. The similar phenotypes are explained by an identical mutation in DMT1 at G185R[154,155]. Deletion of DMT1 also resulted in loss of iron transport by the intestine but not the liver or placenta[156]. The finding that heme is broken down intracellularly and a portion of DMT1 is found inside the enterocyte could suggest that DMT1 is involved in heme-iron absorption. There is an absolute requirement for DMT1 in the uptake of iron by the intestine[156], suggesting that intestinal absorption of iron from heme also requires DMT1 but this remains to be determined (Figure 5).
Intestinal expressed HFE is recognised to regulate iron absorption via the uptake of transferrin bound iron by crypt cells. The finding that HFE is expressed along the terminal web of enterocytes during iron deficiency where it co-localised with DMT1, raises the possibility that HFE may function directly in iron absorption and this may include heme-iron[157]. This is also supported by the finding that HFE expression is inversely proportional to iron absorption[157]. If this is the case then HFE is positioned to interact with HCP1, the putative heme receptor and DMT1. Whether DMT1 and HFE work intracellularly (such as in lysosomes) at levels that cannot be detected by immunofluorescent microscopy remains to be determined.
Basolateral transport of non-heme iron involves ferroportin/Ireg-1/MTP-1/SLC40A1, most often referred to as ferroportin[25]. This is based on the study showing that over-expression of ferroportin in macrophages during erythrophagocytosis increased release of non-heme iron, but not heme[158]. This observation is likely to apply to the enterocyte but this needs to be determined. Also selective deletion of ferroportin in mice resulted in non-heme iron accumulation within enterocytes[159] which provides support for the hypothesis that ferroportin functions with non-heme iron (Figure 5).
Baranano and co-workers have identified a microsomal membrane Fe(II) transporter from the spleen which presumably represents an iron transporter expressed by macrophages. It is induced by heme, and depends on hydrolysable triphosphate, magnesium and tempera-ture[160]. It is proposed that following heme catabolism by macrophages, Fe(II) is shunted into the lumen of the endoplasmic reticulum. Others have found a similar transporter in liver microsomes[161]. Whether this transporter functions in enterocytes remains to be determined.
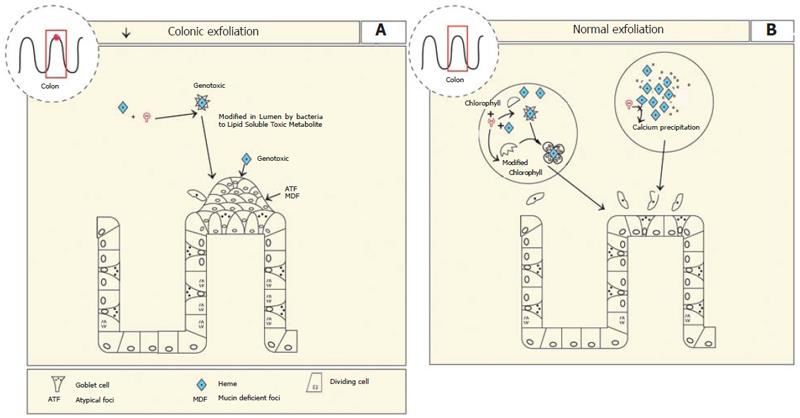
Although heme-iron is more bio-available than non-heme iron it has limited ability to be absorbed. Therefore, unabsorbed heme reaches the colon. Luminal heme is also derived from the blood via extravasation and from desquamation. Previous studies have shown that heme irritates the epithelium of the colon as evidenced by mild diarrhoea[162,163]. It was shown that feeding heme but not non-heme iron to rats results in significant increased proliferation of colonic mucosa[162]. In addition, the incidence of aberrant atypical foci (ATF) and mucin-depleted foci (MDF)[164] increased as the heme content of the diet increased suggesting that heme is carcinogenic[164,165]. In fact, it was demonstrated that heme was genotoxic in the human colon tumour cell line HT29[166].
It has been shown that a heme breakdown product rather than heme or iron per se was responsible for the inflammation and ATF formation[162,163]. In the colon some heme breakdown products are produced by the presence of colonic bacteria[167], and it has been suggested the heme is converted to a cytotoxic factor, although it has not been fully characterised[162,163]. Gene microarray analysis of 365 genes expressed by the colon revealed that feeding heme down-regulated mucosal pentraxin 30-fold[168,169]. Since pentraxin is involved in the recognition and clearance of dying cells, a process that is normally ongoing in the intestinal tract, downregulation of this gene by heme infers that apoptosis of colonic mucosal cells may be inhibited. If this is true then it might explain the increased carcinogenic potential if cells with mutated genomes cannot be eliminated[168]. In support of this, De Vogel et. al., showed that heme supplementation decreased colonic exfoliation[170] (Figure 6).
The cytotoxic affect of heme on the colon was lost when the diet was supplemented with green vegetables[170]. It was hypothesised that chlorophyll in green vegetables inhibited the formation of the heme factor by competing for solubilisation with heme in the large intestine. Alternatively, chlorophyll and heme could form a complex that blocks the site of covalent modification of the heme and reduces the formation of the heme factor[170]. Calcium was also shown to protect against the effects of heme on colonic proliferation and normalising pentraxin expression, presumably because calcium precipitates heme, thereby preventing the formation of the soluble heme induced cytotoxic factor[169,171,172]. This conclusion is consistent with the inhibitory effect that calcium has on heme bioavailability for its absorption in the small intestine[171] (Figure 6A and B).
Peristaltic contractions are controlled by stellate shaped non-neuronal interstitial cells of Cajal (ICC) situated within the myenteric plexus (ICC-MY)[173-177]. Clusters of spindle shaped bipolar ICC found throughout the circular and longitudinal muscle layers (ICC-DMP) generate pacemaker potentials spontaneously but these are modified by neural input[177]. Adjacent to the submucosa and within the circular muscle layer ICC also appear to synapse with nerves (ICC-IM)[174-177]. Loss of ICC leads to markedly impaired neurotransmission and typical gastrointestinal motor patterns indicating their importance in co-ordinating neural modulation of intestinal motility. In the small intestine ICC-MY appear important for pacemaker ICC but in other regions of the bowel this is regulated by ICC-IM.
The network is connected to the smooth muscle syncytium via either gap junctions or peg in socket junctions. These membrane specialisations provide a means of conducting pacemaker currents to intestinal smooth muscle[174-177]. It is thought that pacemaker potentials originate from unitary potentials caused by the release of calcium from mitochondrial stores[177,178] which in turn cause a rise in membrane potential generated by opening of Ca2+ permeable channels. The plateau component observed in pacemaker potentials is generated by opening Ca2+ activated Cl- channels[179]. Repolarisation involves removal of cytosolic Ca2+ to stores and K+ transport via activated K+ channels[179]. The frequency of these events establishes the pacemaker potential of a particular region of the intestine. Muscle contraction will occur providing the membrane potential is capable of activating L-type Ca2+ channels and depolarising the cell[180]. The resulting increase in cytosolic Ca2+ levels is coupled to contraction. Contraction is limited by activation of large-conductance Ca2+-activated K+ channels and L-type Ca2+ inactivation[180].
It has been shown that HO-2 but not HO-1 is present in all classes of ICC (-MY, -IM & -DMP), although HO-2 expression was greater in ICC-MY than in ICC-IM. Enteric neurons also express HO-2[180-192]. In the gastric fundus and in particular mucosal epithelial cells, neurons of the submucosal and myenteric plexus and ICC co-express HO-2 and BVR indicating that these cells have the capacity to generate bilirubin[47]. Since ICC have numerous mitochondria it is hypothesised they produce heme to serve as substrate for HO-2 activity and the CO produced may regulate membrane potential and in turn affect intestinal contraction[186]. In the genetic absence of ICC and in HO-2 knockout mice the membrane potential of intestinal smooth muscle is depolarised compared with wild type controls[174,185,188]. Studies have shown that the HO-2 mediated hyperpolarisation is probably due to the effect of CO on activation of K+ currents in smooth muscle[181,184], and that exogenous CO given to HO-2 knockout mice hyperpolarises the resting membrane potential[191]. Supporting this, the membrane is more hyperpolarised near the submucosa and these cells have higher HO-2 activity and CO production than cells near the myenteric plexus where the membrane is more depolarised[191]. Taken together it suggests that CO produced from ENS and ICC function in maintaining membrane potential and the gradient that exists along the longitudinal and across the circular musculature[184,191]. It would be expected that increased CO production would result in a greater level of smooth muscle relaxation because the membrane potential is further away from threshold. The mechanism by which CO reduces the resting membrane potential is unclear[188].
Within the intestine heme serves important roles in energy production, in enzymes involved in detoxification, in the generation of the second messenger gases NO and CO and the antioxidant bilirubin. The products of heme breakdown namely CO and bilirubin restrict oxidative stress, inflammation, and regulate the cell cycle and differentiation in the crypt region. Excess heme may also promote the development towards colon cancer. Dietary heme is an important source of iron for the body and the absorption of iron from heme differs from non-heme. The molecular mechanism operating in the early phases of absorption appears to involve a transporter although there is evidence of a receptor mediated process and numerous other proteins may function in heme-iron as in non-heme iron absorption. The ability of HO to perform these varied functions within the enterocyte probably depends on different compartments within the cell which are differentially accessed by heme and HO. Future studies will determine how heme-iron is absorbed and the mechanisms by which HO regulates the cell cycle and differentiation, limits the inflammatory process.
The authors thank Trevor Redgrave, Umbreen Uhmed and Denise Tomizzi for constructive comments during the writing of this review. The artistic contribution by Joanna Lamb is gratefully appreciated.
| 1. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 517] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 1998;78:993-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557-2568. [PubMed] |
| 4. | Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 339] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 608] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Karibian D, London IM. Control of heme synthesis by feedback inhibition. Biochem Biophys Res Commun. 1965;18:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Lebrón JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, Feder JN, Bjorkman PJ. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 456] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Oates PS, Morgan EH. Effects of dietary iron loading with carbonyl iron and of iron depletion on intestinal growth, morphology, and expression of transferrin receptor in the rat. Anat Rec. 1996;246:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Oates PS, Morgan EH. Ferritin gene expression and transferrin receptor activity in intestine of rats with varying iron stores. Am J Physiol. 1997;273:G636-G646. [PubMed] |
| 10. | Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388-6394. [PubMed] |
| 11. | Tenhunen R, Marver HS, Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970;75:410-421. [PubMed] |
| 12. | Tenhunen R, Marver H, Pimstone NR, Trager WF, Cooper DY, Schmid R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry. 1972;11:1716-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Schuller DJ, Wilks A, Ortiz de Montellano PR, Poulos TL. Crystal structure of human heme oxygenase-1. Nat Struct Biol. 1999;6:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Shibahara S, Müller R, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985;82:7865-7869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 332] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411-419. [PubMed] |
| 16. | Alam J, Cai J, Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5' sequences are required for induction by heme or heavy metals. J Biol Chem. 1994;269:1001-1009. [PubMed] |
| 17. | Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G964-G971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Maines MD, Kappas A. Metals as regulators of heme metabolism. Science. 1977;198:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 226] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Rosenberg DW, Kappas A. Induction of heme oxygenase in the small intestinal epithelium: a response to oral cadmium exposure. Toxicology. 1991;67:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Takahashi S, Takahashi Y, Yoshimi T, Miura T. Oxygen tension regulates heme oxygenase-1 gene expression in mammalian cell lines. Cell Biochem Funct. 1998;16:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993;268:14678-14681. [PubMed] |
| 22. | Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci U S A. 1991;88:5364-5368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 187] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919-10924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 820] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 24. | Bissell DM, Guzelian PS. Degradation of endogenous hepatic heme by pathways not yielding carbon monoxide. Studies in normal rat liver and in primary hepatocyte culture. J Clin Invest. 1980;65:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1035] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 26. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2671] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 27. | Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1013] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 28. | Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 439] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925-10930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1062] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 31. | McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 743] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 32. | Cable JW, Cable EE, Bonkovsky HL. Induction of heme oxygenase in intestinal epithelial cells: studies in Caco-2 cell cultures. Mol Cell Biochem. 1993;129:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Follett JR, Suzuki YA, Lönnerdal B. High specific activity heme-Fe and its application for studying heme-Fe metabolism in Caco-2 cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1125-G1131. [PubMed] |
| 34. | Yu HP, Choudhry MA, Shimizu T, Hsieh YC, Schwacha MG, Yang S, Chaudry IH. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of estrogen receptor-{beta}-dependent HO-1. J Leukoc Biol. 2006;79:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Abbasoğlu SD, Erbil Y, Eren T, Giriş M, Barbaros U, Yücel R, Olgaç V, Uysal M, Toker G. The effect of heme oxygenase-1 induction by octreotide on radiation enteritis. Peptides. 2006;27:1570-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Coëffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotté P, Déchelotte P. Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr. 2002;132:2570-2573. [PubMed] |
| 37. | Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology. 1998;114:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Hartmann F, Owen R, Bissell DM. Characterization of isolated epithelial cells from rat small intestine. Am J Physiol. 1982;242:G147-G155. [PubMed] |
| 39. | Uc A, Britigan BE. Does heme oxygenase-1 have a role in Caco-2 cell cycle progression. Exp Biol Med (Maywood). 2003;228:590-595. [PubMed] |
| 40. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] |
| 41. | Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 764] [Cited by in RCA: 793] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 42. | Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rümmele P, Farkas S, Schölmerich J. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol. 2005;140:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Salim M, Brown-Kipphut BA, Maines MD. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem. 2001;276:10929-10934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc Natl Acad Sci U S A. 2005;102:7109-7114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916-19923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda). 2005;20:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Colpaert EE, Timmermans JP, Lefebvre RA. Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus. Free Radic Biol Med. 2002;32:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Wang TG, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. FASEB J. 2000;14:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Baker SS, Baker RD Jr. Antioxidant enzymes in the differentiated Caco-2 cell line. In Vitro Cell Dev Biol. 1992;28A:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, Berg CL, Sassa S, Wood BL, Abkowitz JL. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 52. | Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218-24225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 500] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 53. | Uc A, Stokes JB, Britigan BE. Heme transport exhibits polarity in Caco-2 cells: evidence for an active and membrane protein-mediated process. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1150-G1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Raffin SB, Woo CH, Roost KT, Price DC, Schmid R. Intestinal absorption of hemoglobin iron-heme cleavage by mucosal heme oxygenase. J Clin Invest. 1974;54:1344-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Dawson JR, Bridges JW. Conjugation and excretion of metabolites of 7-hydroxycoumarin in the small intestine of rats and guinea-pigs. Biochem Pharmacol. 1979;28:3291-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Rosenberg DW, Kappas A. Characterization of heme oxygenase in the small intestinal epithelium. Arch Biochem Biophys. 1989;274:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Thomas FB, McCullough FS, Greenberger NJ. Effect of phenobarbital on the absorption of inorganic and hemoglobin iron in the rat. Gastroenterology. 1972;62:590-599. [PubMed] |
| 58. | Böni RE, Huch Böni RA, Galbraith RA, Drummond GS, Kappas A. Tin-mesoporphyrin inhibits heme oxygenase activity and heme-iron absorption in the intestine. Pharmacology. 1993;47:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 958] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 60. | Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52:1788-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | Kivistö KT, Niemi M, Fromm MF. Functional interaction of intestinal CYP3A4 and P-glycoprotein. Fundam Clin Pharmacol. 2004;18:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Shimizu M, Lasker JM, Tsutsumi M, Lieber CS. Immunohistochemical localization of ethanol-inducible P450IIE1 in the rat alimentary tract. Gastroenterology. 1990;99:1044-1053. [PubMed] |
| 63. | Kaminsky LS, Fasco MJ. Small intestinal cytochromes P450. Crit Rev Toxicol. 1991;21:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Hoensch H, Woo CH, Raffin SB, Schmid R. Oxidative metabolism of foreign compounds in rat small intestine: cellular localization and dependence on dietary iron. Gastroenterology. 1976;70:1063-1070. [PubMed] |
| 65. | Pascoe GA, Sakai-Wong J, Soliven E, Correia MA. Regulation of intestinal cytochrome P-450 and heme by dietary nutrients. Critical role of selenium. Biochem Pharmacol. 1983;32:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Dubey RK, Singh J. Localization and characterization of drug-metabolizing enzymes along the villus-crypt surface of the rat small intestine--I. Monooxygenases. Biochem Pharmacol. 1988;37:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Traber PG, Wang W, Yu L. Differential regulation of cytochrome P-450 genes along rat intestinal crypt-villus axis. Am J Physiol. 1992;263:G215-G223. [PubMed] |
| 68. | Kappas A, Drummond GS. Control of heme and cytochrome P-450 metabolism by inorganic metals, organometals and synthetic metalloporphyrins. Environ Health Perspect. 1984;57:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Rosenberg DW, Anderson KE, Kappas A. The potent induction of intestinal heme oxygenase by the organotin compound, bis(tri-n-butyltin)oxide. Biochem Biophys Res Commun. 1984;119:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Rosenberg DW, Kappas A. Actions of orally administered organotin compounds on heme metabolism and cytochrome P-450 content and function in intestinal epithelium. Biochem Pharmacol. 1989;38:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Dhur A, Galan P, Hercberg S. Effects of different degrees of iron deficiency on cytochrome P450 complex and pentose phosphate pathway dehydrogenases in the rat. J Nutr. 1989;119:40-47. [PubMed] |
| 72. | Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 73. | Maines MD. Zinc . protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta. 1981;673:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Anderson KE, Simionatto CS, Drummond GS, Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984;228:327-333. [PubMed] |
| 75. | Posselt AM, Kwong LK, Vreman HJ, Stevenson DK. Suppression of carbon monoxide excretion rate by tin protoporphyrin. Am J Dis Child. 1986;140:147-150. [PubMed] |
| 76. | Rosenberg DW, Drummond GS, Kappas A. The in vitro and in vivo inhibition of intestinal heme oxygenase by tin-protoporphyrin. Pharmacology. 1989;39:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Vreman HJ, Hintz SR, Kim CB, Castillo RO, Stevenson DK. Effects of oral administration of tin and zinc protoporphyrin on neonatal and adult rat tissue heme oxygenase activity. J Pediatr Gastroenterol Nutr. 1988;7:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Vreman HJ, Gillman MJ, Stevenson DK. In vitro inhibition of adult rat intestinal heme oxygenase by metalloporphyrins. Pediatr Res. 1989;26:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | DeSandre GH, Wong RJ, Morioka I, Contag CH, Stevenson DK. The effectiveness of oral tin mesoporphyrin prophylaxis in reducing bilirubin production after an oral heme load in a transgenic mouse model. Biol Neonate. 2006;89:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677-10684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Kappas A, Simionatto CS, Drummond GS, Sassa S, Anderson KE. The liver excretes large amounts of heme into bile when heme oxygenase is inhibited competitively by Sn-protoporphyrin. Proc Natl Acad Sci U S A. 1985;82:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Berglund L, Angelin B, Blomstrand R, Drummond G, Kappas A. Sn-protoporphyrin lowers serum bilirubin levels, decreases biliary bilirubin output, enhances biliary heme excretion and potently inhibits hepatic heme oxygenase activity in normal human subjects. Hepatology. 1988;8:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Drummond GS, Rosenberg DW, Kappas A. Intestinal heme oxygenase inhibition and increased biliary iron excretion by metalloporphyrins. Gastroenterology. 1992;102:1170-1175. [PubMed] |
| 84. | Kappas A, Drummond GS, Galbraith RA. Prolonged clinical use of a heme oxygenase inhibitor: hematological evidence for an inducible but reversible iron-deficiency state. Pediatrics. 1993;91:537-539. [PubMed] |
| 85. | Hartmann F, Bissell DM. Metabolism of heme and bilirubin in rat and human small intestinal mucosa. J Clin Invest. 1982;70:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Kandall SR, Thaler MM, Erickson RP. Intestinal development of lysosomal and microsomal beta glucuronidase and bilirubin uridine diphosphoglucyronyl transferase in normal and jaundiced rats. J Pediatr. 1973;82:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 87. | Chow A, Zhang R. Glutamine reduces heat shock-induced cell death in rat intestinal epithelial cells. J Nutr. 1998;128:1296-1301. [PubMed] |
| 88. | Rhoads JM, Argenzio RA, Chen W, Graves LM, Licato LL, Blikslager AT, Smith J, Gatzy J, Brenner DA. Glutamine metabolism stimulates intestinal cell MAPKs by a cAMP-inhibitable, Raf-independent mechanism. Gastroenterology. 2000;118:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 89. | Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol (1985). 2001;90:2403-2410. [PubMed] |
| 90. | De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Uehara K, Takahashi T, Fujii H, Shimizu H, Omori E, Matsumi M, Yokoyama M, Morita K, Akagi R, Sassa S. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit Care Med. 2005;33:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Mueller T, Podolsky DK. Nucleotide-binding-oligomerization domain proteins and toll-like receptors: sensors of the inflammatory bowel diseases' microbial environment. Curr Opin Gastroenterol. 2005;21:419-425. [PubMed] |
| 93. | Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 94. | Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 95. | Farmer DG, Anselmo D, Da Shen X, Ke B, Carmody IC, Gao F, Lassman C, McDiarmid SV, Shaw G, Busuttil RW. Disruption of P-selectin signaling modulates cell trafficking and results in improved outcomes after mouse warm intestinal ischemia and reperfusion injury. Transplantation. 2005;80:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Yamaguchi T, Shioji I, Sugimoto A, Komoda Y, Nakajima H. Chemical structure of a new family of bile pigments from human urine. J Biochem. 1994;116:298-303. [PubMed] |
| 98. | Kozaki N, Shimizu S, Chijiiwa K, Yamaguchi K, Kuroki S, Shimoharada K, Yamaguchi T, Nakajima H, Tanaka M. Bilirubin as an anti-oxidant for surgical stress: a preliminary report of bilirubin oxidative metabolites. HPB Surg. 1999;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 547] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 100. | Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 230] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 101. | Brüne B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497-504. [PubMed] |
| 102. | Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 313] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 103. | Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 2104] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 104. | Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999;291:416-423. [PubMed] |
| 105. | Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol. 2000;278:H1613-H1617. [PubMed] |
| 106. | Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553-3563. [PubMed] |
| 107. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3189] [Cited by in RCA: 3257] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 108. | Silver BJ, Hamilton BD, Toossi Z. Suppression of TNF-alpha gene expression by hemin: implications for the role of iron homeostasis in host inflammatory responses. J Leukoc Biol. 1997;62:547-552. [PubMed] |
| 109. | Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1735] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 110. | Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 870] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 111. | Moore BA, Otterbein LE, Türler A, Choi AM, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Nakao A, Moore BA, Murase N, Liu F, Zuckerbraun BS, Bach FH, Choi AM, Nalesnik MA, Otterbein LE, Bauer AJ. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol. 2004;556:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 115. | Kubes P. Nitric oxide-induced microvascular permeability alterations: a regulatory role for cGMP. Am J Physiol. 1993;265:H1909-H1915. [PubMed] |
| 116. | Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222-G229. [PubMed] |
| 117. | Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996;270:G225-G237. [PubMed] |
| 118. | Salzman A, Denenberg AG, Ueta I, O'Connor M, Linn SC, Szabó C. Induction and activity of nitric oxide synthase in cultured human intestinal epithelial monolayers. Am J Physiol. 1996;270:G565-G573. [PubMed] |
| 119. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 120. | Hassoun EA, Stohs SJ. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology. 1996;112:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Arteel GE, Briviba K, Sies H. Protection against peroxynitrite. FEBS Lett. 1999;445:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 219] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 122. | Guittet O, Decottignies P, Serani L, Henry Y, Le Maréchal P, Laprévote O, Lepoivre M. Peroxynitrite-mediated nitration of the stable free radical tyrosine residue of the ribonucleotide reductase small subunit. Biochemistry. 2000;39:4640-4648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem. 2002;91:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 333] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 124. | Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 125. | Hartsfield CL, Alam J, Cook JL, Choi AM. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol. 1997;273:L980-L988. [PubMed] |
| 126. | Cavicchi M, Gibbs L, Whittle BJ. Inhibition of inducible nitric oxide synthase in the human intestinal epithelial cell line, DLD-1, by the inducers of heme oxygenase 1, bismuth salts, heme, and nitric oxide donors. Gut. 2000;47:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 127. | Bezwoda WR, Bothwell TH, Charlton RW, Torrance JD, MacPhail AP, Derman DP, Mayet F. The relative dietary importance of haem and non-haem iron. S Afr Med J. 1983;64:552-556. [PubMed] |
| 128. | Carpenter CE, Mahoney AW. Contributions of heme and nonheme iron to human nutrition. Crit Rev Food Sci Nutr. 1992;31:333-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Callender ST, Mallett BJ, Smith MD. Absorption of haemoglobin iron. Br J Haematol. 1957;3:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 130. | Bannerman RM. Quantitative Aspects Of Hemoglobin-Iron Absorption. J Lab Clin Med. 1965;65:944-950. [PubMed] |
| 131. | Weintraub LR, Conrad ME, Crosby WH. Absorption of hemoglobin iron by the rat. Proc Soc Exp Biol Med. 1965;120:840-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 132. | Conrad ME, Weintraub LR, Sears DA, Crosby WH. Absorption of hemoglobin iron. Am J Physiol. 1966;211:1123-1130. [PubMed] |
| 133. | Wheby MS, Spyker DA. Hemoglobin iron absorption kinetics in the iron-deficient dog. Am J Clin Nutr. 1981;34:1686-1693. [PubMed] |
| 134. | Worthington MT, Cohn SM, Miller SK, Luo RQ, Berg CL. Characterization of a human plasma membrane heme transporter in intestinal and hepatocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1172-G1177. [PubMed] |
| 135. | Vaghefi N, Guillochon D, Bureau F, Neuville D, Lebrun F, Arhan P, Bouglé D. The effect of cysteine and 2,4-dinitrophenol on heme and nonheme absorption in a rat intestinal model. J Nutr Biochem. 2000;11:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | Heinrich HC, Gabbe EE, Kugler G. Comparative absorption of ferri-haemoglobin- 59 Fe-Ferro-haemoglobin- 59 Fe and 59 Fe 3+ - 59 Fe 2+ in humans with normal and depleted iron sotres. Eur J Clin Invest. 1971;1:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 137. | Gabbe EE, Heinrich HC, Brüggemann J, Pfau AA. Iron absorption from hemiglobin (stable oxidation product of hemoglobin) in relation to the dose in subjects with normal and depleted iron stores. Nutr Metab. 1979;23:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 138. | Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC. Identification of an intestinal heme transporter. Cell. 2005;122:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 498] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 139. | Gräsbeck R, Kouvonen I, Lundberg M, Tenhunen R. An intestinal receptor for heme. Scand J Haematol. 1979;23:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Tenhunen R, Gräsbeck R, Kouvonen I, Lundberg M. An intestinal receptor for heme: its parital characterization. Int J Biochem. 1980;12:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Gräsbeck R, Majuri R, Kouvonen I, Tenhunen R. Spectral and other studies on the intestinal haem receptor of the pig. Biochim Biophys Acta. 1982;700:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 142. | Roberts SK, Henderson RW, Young GP. Modulation of uptake of heme by rat small intestinal mucosa in iron deficiency. Am J Physiol. 1993;265:G712-G718. [PubMed] |
| 143. | Alam J, Smith A. Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J Biol Chem. 1989;264:17637-17640. [PubMed] |
| 144. | Majuri R, Gräsbeck R. A rosette receptor assay with haem-microbeads. Demonstration of a haem receptor on K562 cells. Eur J Haematol. 1987;38:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Majuri R. Heme-binding plasma membrane proteins of K562 erythroleukemia cells: adsorption to heme-microbeads, isolation with affinity chromatography. Eur J Haematol. 1989;43:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Galbraith RA, Sassa S, Kappas A. Heme binding to murine erythroleukemia cells. Evidence for a heme receptor. J Biol Chem. 1985;260:12198-12202. [PubMed] |
| 147. | Galbraith RA. Heme binding to Hep G2 human hepatoma cells. J Hepatol. 1990;10:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Parmley RT, Barton JC, Conrad ME, Austin RL, Holland RM. Ultrastructural cytochemistry and radioautography of hemoglobin--iron absorption. Exp Mol Pathol. 1981;34:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 149. | Wyllie JC, Kaufman N. An electron microscopic study of heme uptake by rat duodenum. Lab Invest. 1982;47:471-476. [PubMed] |
| 150. | Bungert HJ. Absorption of hemoglobin and hemoglobin iron in alcohol-induced liver injury. Digestion. 1973;9:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 151. | Higa Y, Oshiro S, Kino K, Tsunoo H, Nakajima H. Catabolism of globin-haptoglobin in liver cells after intravenous administration of hemoglobin-haptoglobin to rats. J Biol Chem. 1981;256:12322-12328. [PubMed] |
| 152. | Bowen BJ, Morgan EH. Anemia of the Belgrade rat: evidence for defective membrane transport of iron. Blood. 1987;70:38-44. [PubMed] |
| 153. | Oates PS, Morgan EH. Defective iron uptake by the duodenum of Belgrade rats fed diets of different iron contents. Am J Physiol. 1996;270:G826-G832. [PubMed] |
| 154. | Fleming MD, Trenor CC 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383-386. [PubMed] |
| 155. | Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 714] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 156. | Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258-1266. [PubMed] |
| 157. | West AR, Thomas C, Sadlier J, Oates PS. Haemochromatosis protein is expressed on the terminal web of enterocytes in proximal small intestine of the rat. Histochem Cell Biol. 2006;125:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 158. | Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 159. | Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 950] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 160. | Barañano DE, Wolosker H, Bae BI, Barrow RK, Snyder SH, Ferris CD. A mammalian iron ATPase induced by iron. J Biol Chem. 2000;275:15166-15173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 161. | Takeda K, Soeda S, Arai M, Kawamura M. ATP hydrolytic activity of an iron-stimulated P-type ATPase of mouse liver microsomes. J UOEH. 2000;22:317-324. [PubMed] |
| 162. | Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Res. 1999;59:5704-5709. [PubMed] |
| 163. | Sesink AL, Termont DS, Kleibeuker JH, Van Der Meer R. Red meat and colon cancer: dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis. 2000;21:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 164. | Pierre F, Taché S, Petit CR, Van der Meer R, Corpet DE. Meat and cancer: haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis. 2003;24:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 165. | Pierre F, Freeman A, Taché S, Van der Meer R, Corpet DE. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. J Nutr. 2004;134:2711-2716. [PubMed] |
| 166. | Glei M, Klenow S, Sauer J, Wegewitz U, Richter K, Pool-Zobel BL. Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes. Mutat Res. 2006;594:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 167. | Young GP, St John DJ, Rose IS, Blake D. Haem in the gut. Part II. Faecal excretion of haem and haem-derived porphyrins and their detection. J Gastroenterol Hepatol. 1990;5:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 168. | Van Der Meer-Van Kraaij C, Van Lieshout EM, Kramer E, Van Der Meer R, Keijer J. Mucosal pentraxin (Mptx), a novel rat gene 10-fold down-regulated in colon by dietary heme. FASEB J. 2003;17:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 169. | van der Meer-van Kraaij C, Kramer E, Jonker-Termont D, Katan MB, van der Meer R, Keijer J. Differential gene expression in rat colon by dietary heme and calcium. Carcinogenesis. 2005;26:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 170. | de Vogel J, Jonker-Termont DS, van Lieshout EM, Katan MB, van der Meer R. Green vegetables, red meat and colon cancer: chlorophyll prevents the cytotoxic and hyperproliferative effects of haem in rat colon. Carcinogenesis. 2005;26:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 171. | Hallberg L, Rossander-Hulthén L, Brune M, Gleerup A. Inhibition of haem-iron absorption in man by calcium. Br J Nutr. 1993;69:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 172. | Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R. Red meat and colon cancer: dietary haem-induced colonic cytotoxicity and epithelial hyperproliferation are inhibited by calcium. Carcinogenesis. 2001;22:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 173. | Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol. 1986;372:501-520. [PubMed] |
| 174. | Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 749] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 175. | Thuneberg L, Peters S. Toward a concept of stretch-coupling in smooth muscle. I. Anatomy of intestinal segmentation and sleeve contractions. Anat Rec. 2001;262:110-124. [PubMed] [DOI] [Full Text] |
| 176. | Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602-G611. [PubMed] |
| 177. | Shea-Donohue T, Cook IJ, de Giorgio R, Tonini M, Dent J, Costa M, Grundy D, Sanders KM, Schemann M, Smith TK. A teaching module on irritable bowel syndrome. Neurogastroenterol Motil. 2005;17 Suppl 3:20-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 178. | Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 179. | Holm AN, Rich A, Sarr MG, Farrugia G. Whole cell current and membrane potential regulation by a human smooth muscle mechanosensitive calcium channel. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1155-G1161. [PubMed] |
| 180. | Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, Chatterjee S, Szurszewski JH, Shah VH, Farrugia G. Carbon monoxide activates human intestinal smooth muscle L-type Ca2+ channels through a nitric oxide-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2005;288:G7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 181. | Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:G799-G804. [PubMed] |
| 182. | Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U S A. 1996;93:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 183. | Ny L, Alm P, Larsson B, Andersson KE. Morphological relations between haem oxygenases, NO-synthase and VIP in the canine and feline gastrointestinal tracts. J Auton Nerv Syst. 1997;65:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 184. | Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol. 1998;274:G350-G358. [PubMed] |
| 185. | Donat ME, Wong K, Staines WA, Krantis A. Heme oxygenase immunoreactive neurons in the rat intestine and their relationship to nitrergic neurons. J Auton Nerv Syst. 1999;77:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 186. | Farrugia G, Szurszewski JH. Heme oxygenase, carbon monoxide, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 187. | Porcher C, Orsoni P, Berdah S, Monges G, Mazet B. Distribution of heme oxygenase 2 in nerves and c-kit(+) interstitial cells in human stomach. Histochem Cell Biol. 1999;112:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 188. | Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci U S A. 2000;97:1851-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 189. | Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 190. | Chen Y, Lui VC, Sham MH, Tam PK. Distribution of carbon monoxide-producing neurons in human colon and in Hirschsprung's disease patients. Hum Pathol. 2002;33:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 191. | Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567-8570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 192. | Piotrowska AP, Solari V, de Caluwé D, Puri P. Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J Pediatr Surg. 2003;38:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
S- Editor Liu Y E- Editor Liu Y