Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4064
Revised: September 5, 2005
Accepted: September 10, 2005
Published online: July 7, 2006
AIM: To characterize the intestinal transport and mechanism of metformin in rats and to investigate whether or not metformin is a substrate for P-glycoprotein (P-gp).
METHODS: The effective intestinal permeability of metformin was investigated using single-pass intestinal perfusion (SPIP) technique in male Waster rats. SPIP was performed in three isolated intestinal segments (duodenum, jejunum and ileum) at the same concentration of metformin (50 μg/mL) to test if the intestinal transport of metformin exhibited site-dependent changes, and in a same isolated intestinal segment (duodenal segment) at three different concentrations of metformin (10, 50, 200 μg/mL) to test if the intestinal transport of metformin exhibited concentration-dependent changes. Besides, P-gp inhibitor verapamil (400 μg/mL) was co-perfused with metformin (50 μg/mL) in the duodenum segment to find out if the intestinal absorption of metformin was affected by P-gp exiting along the gastrointestinal track. Stability studies were conducted to ensure that the loss of metformin could be attributed to intestinal absorption.
RESULTS: The effective permeability values (Peff) of metformin in the jejunum and ileum at 50 μg/mL were significantly lower than those in the duodenum at the same concentration. Besides, Peff values in the duodenum at high concentration (200 μg/mL) were found to be significantly lower than those at low and medium concentrations (10 and 50 μg/mL). Moreover the co-perfusion with verapamil did not increase the Peff value of metformin at 50 μg/mL in the duodenum.
CONCLUSION: Metformin could be absorbed from the whole intestine, with the main absorption site at duodenum. This concentration-dependent permeability behavior in the duodenum indicates that metformin is transported by both passive and active carrier-mediated saturable mechanism. The Peff value can not be increased by co-perfusion with verapamil, indicating that absorption of metformin is not efficiently transported by P-gp in the gut wall. Furthermore metformin is neither a substrate nor an inducer of P-gp. Based on the Peff values obtained in the present study and using established relationships, the human fraction dose absorbed for metformin is estimated to be 74%-90% along human intestine.
- Citation: Song NN, Li QS, Liu CX. Intestinal permeability of metformin using single-pass intestinal perfusion in rats. World J Gastroenterol 2006; 12(25): 4064-4070
- URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4064.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4064
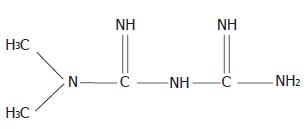
Metformin (dimethylbiguanide), N,N-dimethylimidodicar-bonimidic biamide (Figure 1) is an oral antihyperglycaemic agent used in the management of non-insulin-dependent diabetes mellitus (NIDDM). It reduces blood glucose levels predominantly by improving hepatic and peripheral tissue sensitivity to insulin without affecting the secretion of this hormone. Metformin also appears to have potentially beneficial effects on serum lipid levels and fibrinolytic activity, although the long-term clinical implications of these effects are unclear[1]. Gastrointestinal absorption of metformin is incomplete, 20%-30% of an oral dose is recovered in the faeces[2]. Absorption is estimated to be complete within 6 h of administration and is presumably confined to the upper part of the intestine[2,3]. Metformin is poorly absorbed from the stomach and the delivery process highly correlated with the rate-limiting factor for metformin absorption from the duodenum, while the whole intestine is necessary for sufficient absorption of drugs[3]. Proportionately more drugs are absorbed after 0.5 g dose than 1.5 g dose, possibly because of the involvement of an active, saturable absorption process[3]. In clinical practice, metformin is an effective antihyperglycaemic agent with additional beneficial effects on metabolic and fibrinolytic variables, both of which can be used as monotherapy or in combination with other antihyperglycaemic agents[1].
The SPIP technique is performed in anesthetized rats whereby a section of intestine is isolated and perfused with a solution of the compound of interest. In the SPIP experimental procedure modifications can be made to the flow rate, length of perfused intestine, and concentration of the compound of interest thus giving the investigator exquisite control of the factors influencing the intestinal absorption of a chemical. In the most basic SPIP protocol the compound of interest is monitored in the perfusate only but not in the blood. Loss of compound, as determined by the difference between the inlet and outlet concentrations, is attributed to absorption but only after preliminary studies rule out other possible factors. The preliminary studies may consist of stability studies in the buffer (unperfused and blank perfused buffed) and homogenates of the intestine.
In vivo rat studies have also been performed in an attempt to study the role of intestinal P-gp in drug absorption and metabolism. P-gp, a plasma membrane protein of about 170 kDa, has been demonstrated in many normal tissues, including intestinal cells[4,5]. P-gp in the gut wall acts as an efflux transporter of certain drugs and studies in several species including the rat indicate that P-gp may play an important role in limiting drug absorption[6,7]. P-gp, a member of the ATP-binding cassette transporter superfamily (ABCB1) and is located on the apical membrane of intestinal enterocytes where it can actively efflux drugs from the cells back into the intestinal lumen[8]. An increasing number of drugs, including HIV protease inhibitors like indinavir, ritonavir, saquinavir and anti-cancer drugs like pacltaxel, docetaxel, etc, have been reported to be substrates for P-gp[9]. Verapamil, a P-gp substrate, is a competitive inhibitor of intestine P-gp in the rat[10], and is used as a tool for P-gp inhibition[10].
The aim of this study was to characterize and classify the intestinal permeability of metformin in rats using SPIP model, and to predict the intestinal absorption mechanism of metformin in humans as well as to investigate whether metformin is a substrate for P-gp.
The high performance liquid chromatography (HPLC) system consisting of a Shimadzu LC-6A auto solvent delivery module pump was purchased from (Shimadzu, Koyto, Janpan. Gilson 118 UV spectrophotometric detector, SCL-6A system controller, CTO-6A column oven, CKChrom chromatograph data system, syringe pump (BT01-YZ1515-B) were produced of Tianjin Xieda Electronic Co. Ltd (Tianjin, China). Homogenizer was from Shanghai Jinda Biochemical Instrument Factory (Shanghai, China). Heating operating table was from Shanghai No.1 Medication Store (Shanghai, China). Analytical balance (TG328A) and electronic balance (JA2003) were abtained from Shanghai Balance Instrument Factory (Shanghai, China). High speed table centrifuge (TGL-16) was purchased from Shanghai No.6 Medical Instruments Factory (Shanghai, China). Constant temperature water bath chamber was a produced of Beijing Xicheng Medical Instruments Factory (Beijing, China).
Metformin (purity: 99.5%) and verapamil (purity: 99.5%) were produced by Department of Pharmaceutical Preparation, Tianjin Institute of Pharmaceutical Research (Tianjin, China). Doxofylline was produced by Research Center for New Drug Evaluation, Tianjin Institute of Pharmaceutical Research (Tianjin, China). Urethane was purchased from Beijing Chemical Engineering Plant (Beijing, China). Phenol red and ammonium acetate (analytical grade) were purchased from Tianjin Chemical Reagent No.1 Plant (Tianjin, China). Ion pair reagent, 1-octanesulfonic acid sodium salt (IPR-B8, 0.25 mol/L) was purchased from Tianjin Chemical Reagent No.2 Plant (Tianjin, China). Methanol (HPLC grade) was purchased from Tianjin Concoct Chemical Reagent Company (Tianjin, China).
Male Waster rats weighting 180-230 g were purchased from Center of Experimental Animals, Tianjin Institute of Pharmaceutical Research (Certificate No. 20050110), Tianjin, China. Animals were acclimated for at least 5 d before the experiments and housed in cage (5 each) under constant temperature (22 ± 2°C) with free access to food and drinking water. Animals were fasted overnight before use either in the SPIP study or for harvesting intestinal tissue to prepare homogenate preparation.
Krebs-Ringe buffer solution[11] (K-R buffer solution containing 7.8 g NaCl, 0.35 g KCl, 1.37 g NaHCO3, 0.02 g MgCl2, 0.22 g NaH2PO4 and glucose in 1.48 g/1000 mL purified water) was used as blank perfusion solution. Phenol red (20 μg/mL) and doxofylline (20 μg/mL) were added to perfusion solution as non-absorbable marker and internal standard, respectively. Perfusion solution containing 10 μg/mL, 50 μg/mL, and 200 μg/mL metformin respectively, 20 μg/mL phenol red and 20 μg/mL doxofylline was used. In addition, 400 μg/mL verapamil was added to perfusion solution containing 50 μg/mL metformin to achieve solution containing P-gp inhibitors.
After an overnight fast, animals were anesthetized with an intraperitoneal (i.p.) injection of urethane (0.7 mL/100 g). Upon verification of the loss of pain reflex, a midline abdominal incision of 3-4 cm was made and a 10-15 cm intestinal segment of interest (duodenum, jejunum and ileum) was isolated and cannulated at both ends with plastic tubing. The segment was first rinsed with 37°C saline to clear the segment, then blank K-R buffer solution (without drug) was pumped into the segment at a constant flow rate of 0.2 mL/min. Blank perfused solution was collected at the outlet and used to prepare metformin solution (10, 50, 200 μg/mL) with doxofylline (20 μg/mL) for stability studies of metformin.
To prepare intestinal homogenates, animals were anesthetized as the above procedure, a 10-15 cm intestinal segment of interest (duodenum, jejunum and ileum) was isolated and removed. The excised intestinal segment was washed with ice-cold buffer and the mucosa was removed from the intestine by scraping the intestine. The intestinal mucosa was weighed and then homogenized with enough ice-cold blank K-R buffer solution to make a 20% w/v homogenate using a homogenizer. The homogenate was then centrifuged for the resultant supernatant, which was used to prepare metformin solution (10, 50, 200 μg/mL) with doxofylline (20 μg/mL) for stability studies of metformin.
SPIP studies were performed using established methods as previously described[12,13]. Male Waster rats weighing 180 g to 230 g were fasted overnight before the perfusion experiment with free access to tap water only. The rats were anesthetized with i.p. injection of urethane (0.7 mL/100 g) and placed on a heating operating table to maintain a body temperature of 37°C. Upon verification of the loss of pain reflex the abdomen was opened by a 3-4 cm midline longitudinal incision of and a 10-15 cm intestinal segment (duodenum, jejunum and ileum) was isolated and cannulated at both ends with plastic tubing. The segment was first rinsed with 37°C saline to clear the segment, and approximately 10 cm of the inlet tubing was placed inside the abdominal cavity to achieve an inlet perfusion solution at 37°C. Drops of saline were added onto the surgical area, which was then was covered with wet pledget to avoid loss of fluid. The experiment was initiated by rapidly filling the segment with perfusion solution and the time was set zero with the immediate start of the perfusion. The perfusion rate was 0.2 mL/min. After approximately 30 min, when steady-state was achieved, the outlet perfused samples were collected on ice at 15 min intervals up to 105 min. Samples were frozen immediately and stored at -20°C. At the end of the experiment, the perfused intestinal segment was measured without stretching.
Thirty rats were divided into 6 groups (n = 5) in the present study. SPIP was performed in different intestinal segments (duodenal, jejunal and ileal segment) to test if the intestinal absorption of metformin could exhibit the characteristics of intestinal site dependence. Each intestinal segment was isolated as follows: duodenum segment beginning from pylorus, jejunum segment beginning from 25 cm away from pylorus, while ileum segment the beginning at the site 20 cm upwards caecum. The above segments were perfused with solution containing 50 μg/mL metformin and 20 μg/mL phenol red. The experimental operation was carried out as described above.
Perfusion solutions of different drug concentrations were perfused to investigate the intestinal absorption mechanism of metformin. Duodenum segment was selected as the perfused segment, perfusion solutions containing 10, 50, 200 μg/mL metformin as well as 20 μg/mL phenol red were perfused to test if the permeability values exhibit concentration-dependent changes.
Duodenum segment was selected for perfusion, 400 μg/mL verapamil was added to the perfusion solution (containing 50 μg/mL metformin and 20 μg/mL phenol red) as an inhibitor of P-gp exiting along the gastrointestinal track, then the changes of metformin absorption were determined to find out if the intestinal absorption of metformin was affected by P-gp exiting along the gastrointestinal track.
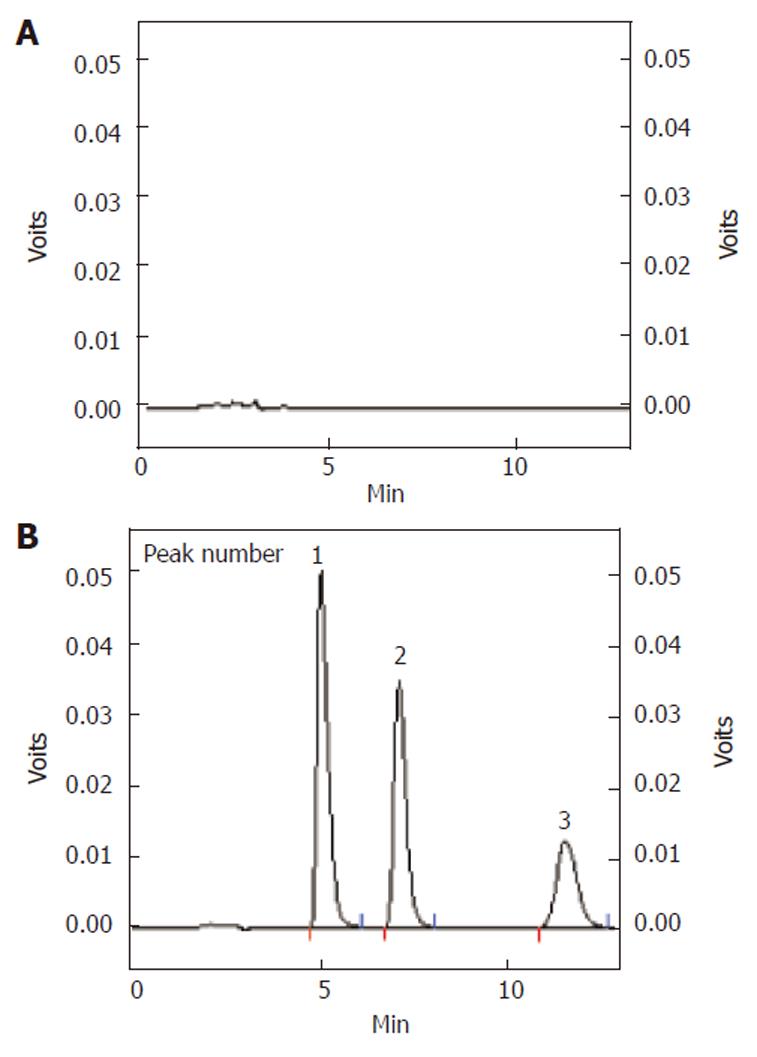
The absorption and stability of samples were analyzed by the HPLC system at UV 250 nm with the oven temperature at 40°C. Analytes were separated on a DiamonsilTM ODS C18 column (5 μm, 200 mm × 4.6 mm I.D.). The mobile phase was made up of methanol: 20 mmol/L NH4AC (containing 1% 0.25 mol/L IPR-B8) = 30:70 (V:V). The flow rate was 1.0 mL/min. Doxofylline (20 μg/mL) was used as an internal standard. Retention time for metformin, doxofylline, phenol red was 5.1 min, 7.2 min and 11.6 min respectively. All sample peaks were well separated (Figure 2).
Calculations were based on outlet perfusate steady-state concentrations achieved after approximate 30 min. The steady-state intestinal effective permeability (Peff, cm/s) was calculated according to a parallel tube model[14-16]:
Peff = [ -Qin· ln (Cout/Cin )’] / A (1)
Where Qin is the perfusion flow rate (0.2 mL/min), A is the mass transfer surface area within the intestinal segment assumed to be the area of a cylinder (2πrL) with the length (L) (measured after 45 min) and radius (r) of 0.18 cm[15-17], Cin and Cout are the inlet and fluid-transport-corrected outlet solution concentrations, respectively. The latter was corrected by multiplying the inlet concentration with [phenol red]in/[phenol red]out.
Metformin stock solution (5, 10, 20, 50, 100, 200 μL) of and phenol red stock solution (10, 20, 30, 40, 50, 60 μL) were precisely drawn, then diluted to 1 mL with K-R buffer solution to make corresponding solutions containing metformin and phenol red 5 and 10, 10, 20, 30, 40, 50, 60 μg/mL phenol red, respectively. The solutions were then injected for determination of calibration curve. The regression curve for metformin and phenol was Y = -0.00634 + 0.00599X (r = 0.9998) and Y = -0.00814 + 0.00870 (r = 0.9995), respectively, indicating good linear correlations.
The intraday and interday precision as well as the accuracy were determined with quality control samples, and five replicates each were analyzed on three consequence days. Three concentrations were chosen from the high medium and low range of the standard curve (10, 50, 200 μg/mL for metformin and 20, 40, 60 μg/mL for phenol red). Precision was expressed as the relative standard deviation (RSD%). Accuracy was expressed as the mean relative error (RE%). A precision (RSD%) less than or equal to 15% and a accuracy (RE%) less than or equal to 15% were accepted. The data are summarized in Table 1.
| Concentration(μg/mL) | Intra-d (n = 5) | Inter-d (n = 5) | ||
| Accuracy Mean RE % | Precision CV % | Accuracy Mean RE % | Precision CV % | |
| Metformin | ||||
| 10 | -0.9 | 3.2 | -3.5 | 4.2 |
| 50 | 1.5 | 1 | 6.8 | 6.3 |
| 200 | 0.9 | 2.1 | 10.5 | 0.7 |
| Phenol red | ||||
| 20 | -2.4 | 3.8 | 7.8 | 6.4 |
| 40 | -1.4 | 2.1 | 6.6 | 6.1 |
| 60 | 1.6 | 3.1 | 14.4 | 3.3 |
The absolute recovery was determined at three concentrations (10, 50, 200 μg/mL for metformin, and 20, 40, 60 μg/mL for phenol red) (n = 6 at each concentration). Results showed that the absolute recovery of metformin at these three concentrations ranged from 97.6 ± 3.73% to 101.6 ± 3.14%, with RSD% less than 3.83%.
The stability study consisted of stability studies in the K-R buffer solutions (unperfused and blank perfused) as well as in homogenates of the intestine. The stability of metfoemin was based on the decrease of the parent compound after 2 h incubation in 37°C water bath as quantitated by HPLC. The stability was determined at concentration of 50 μg/mL metformin, and 40 μg/mL phenol red, 100.8 ± 0.74% and 108.1 ± 3.22% metformin remained after perfusion with unperfused and perfused blank K-R buffer for 2 h. For the perfusion with intestinal homogenate, 2 h incubation at least 87.5 ± 0.69% metformin remained in the homogenate (101.9% ± 1.74%, 87.5% ± 0.69% and 90.0% ± 1.75% for duodenal, jejunal, ileal homogenate, respectively). Results demonstrated that in the stability study, metformin was sufficiently stable (> 80% remaining) for the duration of the SPIP experiment (90 min) in K-R buffer solution, perfused K-R buffer solution and intestinal homogenates.
The results reported were expressed as means±SD. The statistical difference between treatment groups was evaluated using analysis of variance (ANOVA) and the identification of significance was carried out with LSD post hoc test. P < 0.05 was considered statistically different.
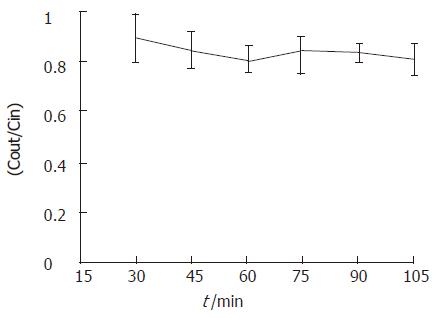
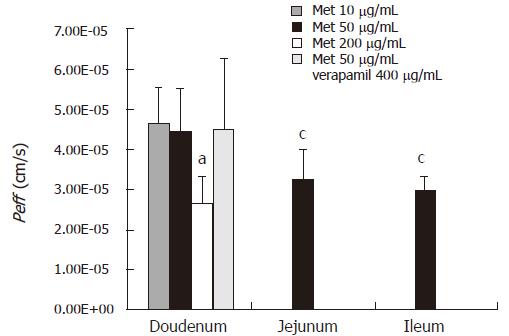
Intestinal permeability of metformin was determined in rat intestine segments (duodenum, jejunum and ileum) using in situ single-pass perfusion technique and the samples were analyzed by the proposed method. Effective permeability values were calculated from the steady-state concentrations of compounds in the perfusate collected from the outlet. Steady-state was confirmed by the ratio of the outlet to inlet concentrations (corrected for water transport) versus time. Representative results are shown in Figure 3. The effective permeability values for each intestinal segment at each concentration are plotted in Figure 4.
Intestinal absorption site dependent changes in permeability were found in the jejum and ileum where the permeability at 50 μg/mL of metformin [(3.26 ± 0.73) × 105 and (2.96 ± 0.36) × 105 cm/s, respectively] was significantly different from those in the duodenum at the same concentration [(4.51 ± 1.08) × 105 cm/s, P < 0.05]. Furthermore, concentration dependent changes in permeability were also found in the duodenum where the permeability at 200 μg/mL of methformin [(2.70 ± 0.63) × 105 cm/s)] was significantly different from that at 10 μg/mL of methformin (4.71 ± 0.86) × 105 cm/s and 50 μg/mL of methformin (4.51 ± 1.08) × 105 cm/s, P < 0.05).
The P-gp efflux inhibitor, verapamil did not affect the measured duodenum Peff for metformin at 50 μg/mL. No significant increase in permeability was found after co-perfusion with verapamil (400 μg/mL) in duodenum [(4.51 ± 1.08) × 105 cm/s for metformin at 50 μg/mL and 4.53 ± 1.75 × 10-5 cm/s for co-perfusion with 400 μg/mL verapamil].
Drugs can cross membranes either by passive processes or by mechanisms involving the active participation of components of the membrane. In the former the drug molecule usually penetrates by passive diffusion along a concentration gradient by virtue of its solubility in the lipid bilayer. The two most common ways for the absorption of drugs are passive transfer by diffusion across the liquid membranes and passive diffusion through the aqueous pores at the tight junctions between cells, which are referred to as transcellular and paracellular absorption, respectively. Transcellular absorption is the predominant pathway for more lipophilic molecules[18]. In contrast, the paracellular route of absorption is limited to small, hydrophilic compounds[19]. Active transport (carrier-mediated membrane transport) is characterized by selectivity, competitive inhibition by congeners, a requirement for energy, saturability, and movement against an electrochemical gradient. Intestinal transport of a compound commonly employs a combination of several mechanisms. The relative importance of each pathway depends on a variety of physicochemical and physiological conditions.
The intestinal permeability is the propensity of a compound to move across the epithelial barrier of the intestine. In vitro and in situ absorption models, such as in situ perfusion of rat intestine, Caco-2 cell monolayer model, and excised intestinal segments in the Ussing chamber, are commonly used to investigate transport mechanism, classify permeability and in vivo absorption of drugs in humans[20,21]. Comparisons between human Peff data and these preclinical permeability models showed that they could be used to investigate and classify passive transport with a high accuracy[22]. Recently, a GI-transit-absorption model (GITA) has been developed to analyze and predict drug absorption, and there is some evidence that this model is very useful for estimating the absorption kinetics of drugs with various characteristics as well as the human data[23].
Among the various models, cell-based assays using Caco-2 and MDCK cell lines are commonly used to predict passive absorption and the results obtained are greatly affected by experimental parameters such as pH[24]. In contrast, in situ approaches provide experimental conditions closer to what is encountered following oral administration, with a lower sensitivity to pH variations due to a preserved microclimate above the epithelial cells[25,26]. These techniques maintain an intact blood supply to the intestine, and can be used to estimate the impact of clearance pathways, such as enzymes and transporters, that are present in the gut. Moreover, drug permeability[27], expression of drug metabolizing enzymes and transporters have been shown to vary along the intestinal tract[28-30], which can be investigated using intestinal perfusion of the various regions. In addition, it was recently reported that oral drug absorption in rats and humans is very similar[31]. Thus, it is likely that the intestinal perfusion conducted in rats may give a better prediction of the fraction of oral dose absorbed in humans than in in vitro models.
The SPIP procedure assumes that loss of drug during the perfusion is due to permeation of the intestine, Therefore, determination of the non-absorptive loss of perfused drug is required. Results of preliminary studies have confirmed that the loss of metformin in the SPIP study could be attributed to the intestinal absorption, and the confidence of the calculated permeabilities is high. The intestinal effective permeability represents a direct measurement of the local absorption rate and reflects the transport velocity across the epithelial barrier, expressed as centimeter per second[32]. In the present study, SPIP was performed in three different intestinal segments to test if the intestinal absorption of metformin exhibited site-dependent changes. The results indicated that metformin could be absorbed along the whole intestine with the main absorption site at duodenum, contributing to the drug absorption. Further comparison of permeability values among high, medium and low concentrations in the duodenal segment demonstrated a concentration-dependent change, exhibiting a decrease in permeability at the highest drug concentration. The result suggested that intestinal absorption of metformin was via passive and transcelluar mechanism involving an active saturable process, or via a membrane transport protein in the gut wall, especially in the duodenum.
The present study also demonstrated that metformin could not be used as a substrate for rat intestinal P-gp because permeability values of metformin in the duodenum segment at 50 μg/mL were not increased by co-perfusion of the P-gp inhibitor verapamil, suggesting that metformin cannot be efficiently transported by P-gp in the gut wall, however, a saturable process may be involved in the intestinal transport of metformin. Since the SPIP experimental protocol cannot completely elucidate transport mechanism (i.e. inability to conduct directional transport studies), further studies with cultured cells or isolated tissue are necessary to identify the particular reason for the concentration dependent changes in permeability. Such studies would permit determination of both influx and efflux permeabilities. Experiments with cells transfected with the gene coding for a particular transport would further identify the specific protein involved in the carrier-mediated process.
Effective intestinal permeability estimated from in situ perfused rat intestine has been shown to correlate well with the extent of in vivo absorption in humans after oral administration of carrier-mediated absorbed drugs[33].It was recently reported that special care must be taken for drugs with a carrier-mediated transport mechanism[22]. Furthermore, although in situ perfusion technique is time consuming, it provides a greater correlation with intestinal absorption in humans than Caco-2 and MDCK cell lines. Thus human intestinal permeability values of metformin may be predicted from permeability values obtained in this rat SPIP experiment according to[16]:
Peff,man = 3.6 ·Peff,rat + 0.03 · 10-4 (2)
Based on the predicted permeability value in humans, the fraction absorbed in humans (fa,min) can further be predicted from rats for carrier-mediated absorbed drugs by [34]:
fa, man = 1 – e-[2 ·Peff,man·tres / r · 2.8] (3)
Where tres is the average small intestine transit time, r is radius in humans (assumed to be 3h and 1.75 cm, respectively).
Using equation (3) and the lowest permeability value of metformin obtained in this study (2.70 × 10-5 cm/s in the duodenum at 50 μg/mL of metformin), the permeability value of metformin in humans was estimated to be 1.0 ×10-5 cm/s. Then applying equation to metformin for the lowest permeability value in humans in this study gave an estimated fa, man of 74%, while for the highest one gave an estimated fa, man of 90%, which is in accordance with the previously reported values [2,3].
The successful development of oral drug delivery formulations requires characterization of the intestinal absorption. Before the preparation for oral administration of extended-release and controlled-release system and targeting drug release system in gastrointestinal track, characteristics of drug absorption and transfer in the gastrointestinal track should be carefully studied and well understood. In general, appropriate candidates for extended-release and controlled-release preparations are molecules that have poor colonic absorption but are characterized by better absorption properties at the upper parts of the gastrointestinal tract[16]. Results of the present study showed that metformin could be absorbed in the whole human intestine, with the main absorption site at duodenum. However, it was reported that absorption of metformin in human intestine is incomplete, 20%-30% of an oral dose can be recovered in the faeces. Furthermore, it has been reported that oral absorption of the drug is slower than that of elimination[35]. Therefore special care should be paid to the drug content and drug administration times (maybe 2 times/d) when preparing extended-release and controlled-release formulations in order to maintain the effective blood concentration. The common side effects of metformin therapy could be concluded as gastrointestinal symptoms, such as abdominal discomfort, nausea, and diarrhea that especially occur during the initial weeks of treatment[36]. Employing of extended-release technique could reduce the direct drug stimulation to the gastrointestinal tract, and may reduce the above gastrointestinal symptoms due to the lower peak exposure of intestinal tissue to the drug.
In conclusion, intestinal permeability of metformin is concentration- and site- dependent. Fraction of the oral dose absorbed after oral administration is 74%-90% along the whole human intestine. Intestinal absorption of metformin may be via passive and transcelluar mechanism involving an active, saturable process, or via a membrane transport protein in the gut wall, especially in the duodenum. The observed intestinal permeability values obtained in the SPIP model fit well the pattern observed in previous permeability studies for other structural different drugs in rat intestine.
| 1. | Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs. 1995;49:721-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 242] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235-246. [PubMed] |
| 3. | Vidon N, Chaussade S, Noel M, Franchisseur C, Huchet B, Bernier JJ. Metformin in the digestive tract. Diabetes Res Clin Pract. 1988;4:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 1037] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 5. | Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735-7738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 1929] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 6. | Terao T, Hisanaga E, Sai Y, Tamai I, Tsuji A. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J Pharm Pharmacol. 1996;48:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 672] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1525] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 9. | Williams GC, Sinko PJ. Oral absorption of the HIV protease inhibitors: a current update. Adv Drug Deliv Rev. 1999;39:211-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Saitoh H, Aungst BJ. Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm Res. 1995;12:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Cutler DJ. Theory of the mean absorption time, an adjunct to conventional bioavailability studies. J Pharm Pharmacol. 1978;30:476-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Sinko PJ, Hu P, Waclawski AP, Patel NR. Oral absorption of anti-AIDS nucleoside analogues. 1. Intestinal transport of didanosine in rat and rabbit preparations. J Pharm Sci. 1995;84:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Svensson US, Sandström R, Carlborg O, Lennernäs H, Ashton M. High in situ rat intestinal permeability of artemisinin unaffected by multiple dosing and with no evidence of P-glycoprotein involvement. Drug Metab Dispos. 1999;27:227-232. [PubMed] |
| 14. | Amidon GL, Kou J, Elliott RL, Lightfoot EN. Analysis of models for determining intestinal wall permeabilities. J Pharm Sci. 1980;69:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Komiya I, Park JY, Kamaani A, Ho NFH, Higuchi WI. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int. J. Pharmaceutics. 1980;4:249-262. [RCA] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Fagerholm U, Johansson M, Lennernäs H. Comparison between permeability coefficients in rat and human jejunum. Pharm Res. 1996;13:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 341] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 1057] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 18. | Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7448] [Cited by in RCA: 9716] [Article Influence: 388.6] [Reference Citation Analysis (1)] |
| 19. | Knipp GT, Ho NF, Barsuhn CL, Borchardt RT. Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci. 1997;86:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3597] [Cited by in RCA: 3487] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 21. | Lennernäs H, Nylander S, Ungell AL. Jejunal permeability: a comparison between the ussing chamber technique and the single-pass perfusion in humans. Pharm Res. 1997;14:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Lennernäs H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. J Pharm Pharmacol. 1997;49:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biol Pharm Bull. 2002;25:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 416] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Högerle ML, Winne D. Drug absorption by the rat jejunum perfused in situ. Dissociation from the pH-partition theory and role of microclimate-pH and unstirred layer. Naunyn Schmiedebergs Arch Pharmacol. 1983;322:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Shiau YF, Fernandez P, Jackson MJ, McMonagle S. Mechanisms maintaining a low-pH microclimate in the intestine. Am J Physiol. 1985;248:G608-G617. [PubMed] |
| 27. | Ungell AL, Nylander S, Bergstrand S, Sjöberg A, Lennernäs H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J Pharm Sci. 1998;87:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Hakkak R, Ronis MJ, Badger TM. Effects of enteral nutrition and ethanol on cytochrome P450 distribution in small intestine of male rats. Gastroenterology. 1993;104:1611-1618. [PubMed] |
| 29. | Zhang QY, Wikoff J, Dunbar D, Kaminsky L. Characterization of rat small intestinal cytochrome P450 composition and inducibility. Drug Metab Dispos. 1996;24:322-328. [PubMed] |
| 30. | Makhey VD, Guo A, Norris DA, Hu P, Yan J, Sinko PJ. Characterization of the regional intestinal kinetics of drug efflux in rat and human intestine and in Caco-2 cells. Pharm Res. 1998;15:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Chiou WL, Barve A. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharm Res. 1998;15:1792-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Lennernäs H. Human intestinal permeability. J Pharm Sci. 1998;87:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 329] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Amidon GL, Sinko PJ, Fleisher D. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res. 1988;5:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 227] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Hoffman A, Stepensky D, Lavy E, Eyal S, Klausner E, Friedman M. Pharmacokinetic and pharmacodynamic aspects of gastroretentive dosage forms. Int J Pharm. 2004;277:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 268] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Gusler G, Gorsline J, Levy G, Zhang SZ, Weston IE, Naret D, Berner B. Pharmacokinetics of metformin gastric-retentive tablets in healthy volunteers. J Clin Pharmacol. 2001;41:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
S- Editor Guo SY L- Editor Wang XL E- Editor Liu Y