Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4038
Revised: February 8, 2006
Accepted: February 18, 2006
Published online: July 7, 2006
AIM: To evaluate the antiviral effect of the effective ingredient of Styela plicata in a murine model of hepatitis B virus carrier.
METHODS: HBV-transgenic mice were divided into 3 groups (control group, lamivudine treatment group and the effective ingredient of Styela plicata treatment group) and assigned to receive normal diet, lamivudine or the effective ingredient of Styela plicata for consecutive weeks. Serum hepatitis B surface antigen was detected by enzyme-linked immunosorbent assay (ELISA) method. Serum HBV DNA was detected by real-time polymerase chain reaction (RT-PCR). Serum T helper (h) 1 cytokine interleukin (IL)-2 and Th2 cytokine IL-6 were detected by the quantitative sandwich enzyme immunoassay technique. Another group of HBV-transgenic mice was assigned to receive the effective ingredient of Styela plicata for consecutive weeks. The histology of liver tissue was evaluated before and after treatment.
RESULTS: Twelve weeks after starting the therapy, serum hepatitis B surface antigen was significantly lowered in Styela plicata -treated mice and lamivudine-treated mice compared with the mice receiving normal diet (F12wk = 88.81, P12wk = 0.000 < 0.01). Serum HBV DNA was significantly lowered in Styela plicata -treated mice and lamivudine-treated mice compared with the mice receiving normal diet (F12wk = 20.71, P12wk = 0.000 < 0.01). However, like lamivudine, the effective ingredient of Styela plicata could not inhibit the replication of HBV completely. A rebound phenomenon of hepatitis B surface antigen and HBV DNA in sera could be found 4 wk after withdrawal of medication. Eight weeks after starting the therapy, serum levels before and after Styela plicata treatment of IL-2 were 2.41 ± 0.38 and 10.56 ± 0.78 ng/L, respectively (t8wk = -16.51, P8wk = 0.000 < 0.01). Compared with the serum levels of IL-2 in the normal diet-treated mice (2.48 ± 0.17 ng/L; t8wk = 13.23, P8wk = 0.000 < 0.01). Serum levels before and after Styela plicata treatment of IL-6 were 63.62 ± 6.31 and 54.52 ± 6.22 ng/L, respectively, compared with the serum levels of IL-6 in the normal diet-treated mice (60.84 ± 4.21 ng/L). Histological analysis of liver from Styela plicata-treated HBV-transgenic mice also showed catabatic status in inflammation and hepatitis B surface antigen.
CONCLUSION: Styela plicata may be an effective antiviral medicine in treating chronic hepatitis B.
-
Citation: Wang R, Du ZL, Duan WJ, Zhang X, Zeng FL, Wan XX. Antiviral treatment of hepatitis B virus-transgenic mice by a marine organism,
Styela plicata . World J Gastroenterol 2006; 12(25): 4038-4043 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4038
Chronic hepatitis B virus (HBV) infection is a serious clinical problem because of its worldwide distribution and possible adverse sequelae, such as hepatic decompensation, cirrhosis, and hepatocellular carcinoma (HCC)[1,2]. Although interferon and lamivudine are recommended for patients with chronic liver disease (CLD)[3,4,6], the high cost, adverse side effects, lower levels of efficacy, and the inability to block the progression to HCC have tremendously limited the use of them[5-7].
In the absence of an ideal therapy many chemicals with biological activities are used in patients with chronic hepatitis. Ascidians, an intertidal marine, are known for their capacity to inhibit tumors, bacterium and virus[8-10]. Recent data have indicated that the effective components of the ascidian Styela plicata (Sp) can inhibit the secretion of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in 2.2.15 cells[11].
HBV-transgenic mice (HBV-Tg), which express products of the HBV genome and also show signs of HBV replication, are a suitable animal model to study the process of destruction of hepatocytes, the critical role of antiviral medicines in controlling HBV replication and gene expression[3,12,13]. In the current study, we evaluated the anti-HBV activity of the alcohol extract of Sp by conducting therapeutic trials in four groups of HBV-Tg expressing similar levels of HBV-markers. A highly significant therapeutic effect of Sp was demonstrated. The mechanism underlying the therapeutic role of Sp in anti-HBV therapy needs to be further investigated.
The HBV (adr subtype) transgenic mice (HBV-Tg) were prepared by microinjection of complete 2.0 copy HBV genome into pronuclei of fertilized eggs[14]. The HBV DNA can be expressed and replicated in the transgenic mice. HBV genomic DNA in mouse line could be transmitted to next generations. The transgenic mice are tolerant to HBV gene products and similar to human chronic HBV carrier[14,15]. Their serum HBsAg can be expressed highly, but serum HBeAg is expressed inapparently.
We purchased the HBV-Tg of either sex from the Infectious Diseases Center of 458 Hospital of Chinese PLA at 6 wk of age. All animals were housed with controlled temperature at 28°C ± 2°C and 16:8 h light-dark cycle.
HBV-Tg were divided into three groups. Group 1 (9 mice, the control group) was administrated with normal foodstuff and physiological saline. Group 2 (9 mice, a treatment group) was allowed to receive lamivudine (50 mg/kg per day), normal foodstuff and physiological saline. Group 3 (9 mice, a treatment group) was administrated with the foodstuff containing the effective ingredient of Sp (5 g/kg per day) and physiological saline. Every group was gastrogavaged for 12 wk. The sera of HBV-Tg were collected before administration of drugs, at the 4th, 8th and 12th wk after administration of drugs, and the 4th wk after withdrawal of drugs.
Another group of HBV-Tg (5 mice) were anesthetized by an injection of amobarbital (70 μg/g). Then an abdominal incision was made. Parts of their livers were immediately excised. Then the incisions were closed. After 2-wk feeding with ordinary foodstuff and physiological saline, the mice were assigned to the foodstuff containing the effective ingredient of Sp (5 g/kg per day) and physiological saline for 10 wk and later the livers were obtained with the method described before.
HBsAg in sera was detected quantitatively by the enzyme-linked immunosorbent assay (ELISA) method, as described in commercial kits (Rongsheng Biotech Co., Ltd, Shanghai, China). The HBsAg levels were expressed in optical density (OD) values. All performance strictly followed the manuals of the ELISA kits.
For HBV DNA quantification by real-time polymerase chain reaction (RT-PCR), viral DNA was extracted from serum using viral DNA extraction solution (DaAn Gene Co., Ltd, Guangzhou, China) according to the manufacturer’s instructions. PCR amplification was performed with a set of PCR primers and a probe corresponding to the surface antigen gene of HBV[15]. The PCR primers are: P1, 5’-ATCCTGCTGCTATGCCTCATCTT; P2, 5’-ACAGTGGGGGAAAGCCCTACGAA. The TaqMan probe is 5’-TGGCTCAGTTTACTAGTGCCATTTG. The PCRs were performed according to the manufacturer’s instructions (DaAn Gene Co., Ltd, Guangzhou, China)[16] using a LightCyclerTM (Roche Diagnostics, Germany). The PCR cycling program consisted of an initial denaturing step at 93°C for 2 min, followed by 40 amplification cycles at 93°C for 5 s, 57°C for 45 s. The HBV DNA titers > 1.00 × 103 (1.00E03) copies/mL were considered to be positive.
Sera were collected before administration of drugs, at the 4th, 8th wk after drug administration. Levels of IL-2 and IL-6 in sera were detected by using ELISA kits specific for IL-2 and IL-6 (Senxiong Technology Industry Co., Ltd, Shanghai, China). The assay used the quantitative sandwich enzyme immunoassay technique. All operations strictly followed the manuals of the ELISA kits.
Some of the specimens obtained were fixed with 4% buffered formalin and embedded in paraffin. Then they were stained with haematoxylin and eosin (HE) and examined on a video monitor. The normal and inflamed regions of the specimens were easily discriminated by HE stains. The degree of liver cell degeneration and inflammation was evaluated according to previous method[17].
The other specimens obtained were fixed with 4% buffered formalin and embedded in paraffin. Then they were stained with orcein and examined on a video monitor. The HBsAg could be observed by orcein stains.
The experiment results were analyzed by SPSS12.0 statistical software. The data for the negative rate were expressed as percentage. The data for the concentration of HBV DNA in serum were expressed as mean ± SD. Difference before and after therapy within one group was analyzed using paired t-test. Difference among the groups was evaluated using analysis of variance (One-way ANOVA). Statistical significance was accepted at the level of P < 0.05.
The changes of HBsAg titers are shown in Table 1. The paired t-test showed that the HBV-Tg which responded to lamivudine therapy had reduced serum HBsAg compared with that prior to therapy from wk 4 to wk 12(t4wk = 2.94, P4wk = 0.039 < 0.05; t8wk = 7.37, P8wk = 0.000 < 0.01; t12wk = 13.54, P12wk = 0.000 < 0.01). The HBV-Tg which responded to the effective ingredient of Sp showed reduced level of serum HBsAg from wk 4 to wk 12 significantly (t4wk = 2.89, P4wk = 0.020 < 0.05; t 8wk = 3.61, P8wk = 0.007 < 0.01; t12wk = 11.11, P12wk = 0.000 < 0.01). Although these treatment groups all exhibited a rebound phenomenon 4 wk after withdrawal of medication, they still exhibited a significant lower level of serum HBsAg compared with that prior to therapy(tlami = 2.39, iPlami = 0.043 < 0.05; tSp = 10.15, PSp = 0.000 < 0.01). One-way ANOVA showed that the HBV-Tg which responded to lamivudine had a significant reduced level of serum HBsAg compared with the control group which responded to normal diet from wk 4 to wk 12 (F4wk = 5.47, P4wk = 0.003 < 0.01; F8wk = 11.59, P8wk = 0.000 < 0.01; F12wk = 88.81, P12wk = 0.000 < 0.01); the HBV-Tg which responded to the effective ingredient of Sp showed a significant reduced level of serum HBsAg compared with the control group which responded to normal diet from wk 8 to wk 12 (F8wk = 11.59, P8wk = 0.001 < 0.01; F12wk = 88.81, P12wk = 0.000 < 0.01). Although these treatment groups all exhibited a rebound phenomenon 4 wk after withdrawal of medication, they still exhibited a significant lower level of serum HBsAg compared with the control group which responded to normal diet (F16wk = 14.79, Plami = 0.011 < 0.05, PSp = 0.000 < 0.01).
| Treatment group | OD values | ||||
| 0 wk | 4 wk | 8 wk | 12 wk | 16 wk | |
| Group 1 normal diet recipients (n = 9) | 1.61 ± 0.01 | 1.61 ± 0.01 | 1.62 ± 0.02 | 1.62 ± 0.01 | 1.60 ± 0.02 |
| Group 2 lamivudine recipients (n = 9) | 1.59 ± 0.03 | 1.00 ± 0.21a,d | 0.92 ± 0.15b,d | 0.76 ± 0.08b,d | 1.29 ± 0.11a,d |
| Group 3 Sp recipients (n = 9) | 1.61 ± 0.02 | 1.32 ± 0.08a | 1.01 ± 0.16b,d | 0.82 ± 0.07b,d | 1.05 ± 0.05b,d |
The anti-HBV DNA production is summarized in Table 2. The paired t-test showed that the HBV-Tg which responded to lamivudine therapy had reduced serum HBV DNA compared with the level of HBV DNA prior to therapy from wk 4 to wk 12 (t4wk = 5.76, P4wk = 0.000 < 0.01, t 8wk = 4.23, P8wk = 0.003 < 0.01, t 12wk = 4.00, P12wk = 0.004 < 0.01). The HBV-Tg which responded to the effective ingredient of Sp showed a significant reduced level of serum HBV DNA from wk 8 to wk 12 (t8wk = 2.43, P8wk = 0.041 < 0.05; t 12wk = 3.57, P12wk = 0.007 < 0.01). One-way ANOVA showed that the HBV-Tg which responded to lamivudine had a significant reduced level of serum HBV DNA compared with the control group which responded to normal diet from wk 8 to wk 12 (F8wk = 11.36, P8wk = 0.000 < 0.01; F12wk = 20.71, P12wk = 0.000 < 0.01). The HBV-Tg which responded to the effective ingredient of Sp showed a significant reduced level of serum HBV DNA compared with the control group which responded to normal diet from wk 8 to wk 12 (F8wk = 11.36, P8wk = 0.001 < 0.01; F12wk = 20.71, P12wk = 0.000 < 0.01). Both the two groups exhibited a rebound phenomenon 4 wk after withdrawal of medication.
| Treatment group | Serum HBV DNA (copies/mL) | ||||
| 0 wk | 4 wk | 8 wk | 12 wk | 16 wk | |
| Group 1 normal diet recipients (n = 9) | 1.35E05 ± 3.64E04 | 1.34 E05 ± 3.20 E04 | 1.53 E05 ± 3.10 E04 | 1.30 E05 ± 2.43 E04 | 2.59 E05 ± 9.37 E04 |
| Group 2 lamivudine recipients (n = 9) | 1.20 E05 ± 2.54 E04 | 5.52 E04 ± 1.63 E04b | 2.48 E04 ± 8.12E03b,d | 1.34 E04 ± 4.48 E03b,d | 1.12 E05 ± 2.70 E04 |
| Group 3 Sp recipients (n = 9) | 1.73 E05 ± 4.69 E04 | 8.39 E04 ± 4.32 E04 | 4.07 E04 ± 1.63 E04a,d | 1.19 E04 ± 7.53 E03b,d | 1.91 E05 ± 6.87 E04 |
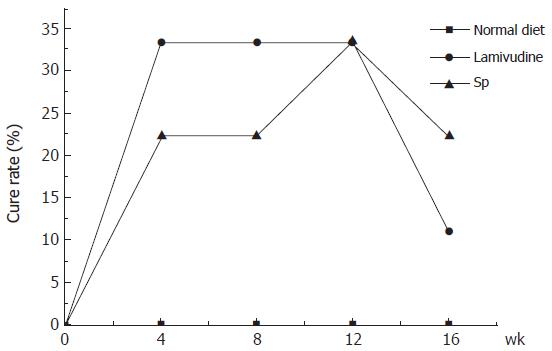
HBV-Tg receiving lamivudine showed a fast cure rate compared with HBV-Tg receiving the effective ingredient of Sp. However, their long-term effects were similar (Figure 1). HBV-Tg receiving either the effective ingredient of Sp or lamivudine exhibited the same cure rate (33%) 12 wk after the start of therapy. Both groups showed a rebound phenomenon 4 wk after withdrawal of medication.
Before treatment, there was no significant difference in the serum levels of IL-2 and IL-6, respectively, between the control group and the treatment group. After weeks of treatment, the paired t-test showed that the HBV-Tg which responded to the effective ingredient of Sp therapy increased serum IL-2 compared with the level of IL-2 prior to therapy from wk 4 to wk 8 (t4wk = -6.74, P4wk = 0.000 < 0.01; t8wk = -16.51, P8wk = 0.000 < 0.01); and the IL-2 serum levels were higher in the Sp-treated group compared with those of the control group (t4wk = 6.58, P4wk = 0.000 < 0.01; t8wk = 13.23, P8wk = 0.000 < 0.01). Although a slight decline could be seen, there was no significant difference in the serum levels of IL-6 before and after treatment (Table 3).
| Treatment group | IL-2 (ng/L) | IL-6 (ng/L) | ||||
| 0 wk | 4 wk | 8 wk | 0 wk | 4 wk | 8 wk | |
| Group 1 normal diet recipients (n = 9) | 2.13 ± 0.23 | 2.33 ± 0.26 | 2.48 ± 0.17 | 63.59 ± 1.82 | 65.40 ± 10.3 | 60.84 ± 4.21 |
| Group 3 Sp recipients (n = 9) | 2.41 ± 0.38 | 7.91 ± 1.09b,-d | 10.56 ± 0.78b,d | 63.62 ± 6.31 | 61.80 ± 4.58 | 54.52 ± 6.22 |
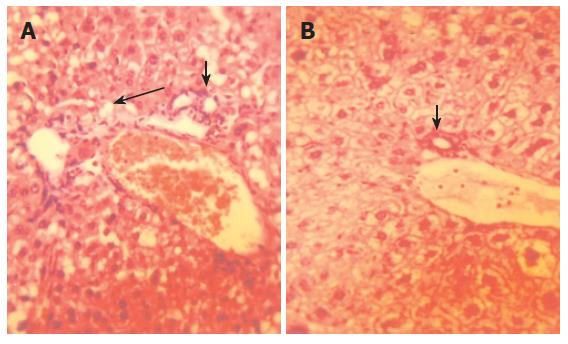
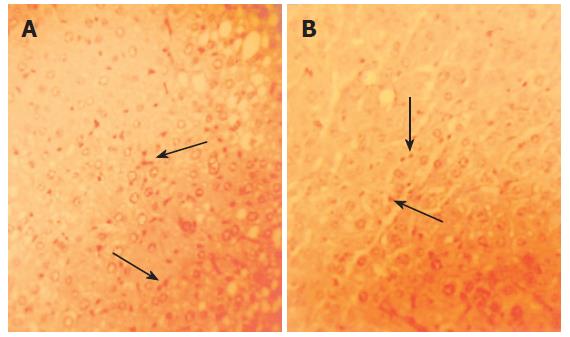
HBV-Tg receiving the effective ingredient of Sp for 10 wk exhibited improvement in inflammation and HBsAg in liver compared with HBV-Tg receiving normal diet (Figures 2 and 3). The semi-quantitative evaluation of inflammatory degree and hepatocytes degeneration degree in mouse liver proved the above findings (Table 4).
| 0 wk | 10 wk | |
| Degeneration degree of hepatocytes | 8.00 ± 0.63 | 5.60 ± 0.68b |
| Inflammation degree of hepatocytes | 5.20 ± 0.37 | 4.00 ± 0.32a |
Ascidians are marine animals with a high ability to synthesize bioactive substances. The extracts of ascidians are most commonly used in antimicrobial assays and anticancer treatments[18-20]. Bioactivity-guided ingredientation of an ethyl acetate extract of the marine ascidian, Pseudodistoma sp., collected in the Tsitsikamma Marine Reserve, revealed that the antimicrobial properties in this extract resided in a group of acyclic amino alcohols, isolated as their peracetylated derivatives[18]. The extract of Eudistoma vannamei Millar, obtained from the northeastern Brazilian coast showed a high toxicity in tumor cell lines tested[20].
Sp is a dominant component of the marine benthos of South China Sea. In recent years, we carried out basic research on the marine organism. The alcohol extracts of the animal have a definite inhibition effect on HBsAg and HBeAg in vitro, although the exact mechanism is not known[11,21,22].
This study suggested that the effective ingredient of Sp might have potential therapeutic value for chronic hepatitis B infection. Thirty-three of the HBV-Tg receiving Sp responded to the therapy and became completely negative for HBV-DNA 12 wk after the start of therapy (Figure 1). The level of serum HBsAg titers was also reduced, though not significantly. But like most present antiviral drugs, the effective ingredient of Sp could not inhibit the replication of HBV completely. Relapse after cessation of the alcohol extract of Sp could be observed, similar to that of lamivudine (Table 1 and Table 2).
In our experiment, we found that the gait and status of psychosis between the effective ingredient of Sp-treated HBV-Tg and the normal diet-treated ones were similar. Histological analysis of mouse liver revealed that the alcohol extract of Sp could ameliorate the inflammation in liver, alleviate hepatocytes degeneration and eliminate HBsAg (Figures 2 and 3, Table 4). Besides, the histological observation showed that there was no distinct toxicity in tissue. However, we did not have further virological/molecular study of HBV markers in the liver. To find more molecular/pharmacological mechanisms of the effect of Sp, we need to measure the HBV DNA and cccDNA levels, as well as HBsAg and HBcAg expression in liver in the following experiments.
Then, we investigated the effects of the effective ingredient of Sp on the serum levels of Th1/Th2 cytokines. It is well known that the identification of functionally distinct CD4+ T helper subpopulations, producing distinct patterns of cytokines, has provided an important insight into the mechanisms by which polarized immune response occurs in vivo in response to pathogens[23]. T helper 1 cytokines, including IFN-g, IL-2 and TNF-b are involved principally in cell-mediated immunity and play a crucial role in the protection from intracellular pathogens, including a number of viruses, while Th2-derived cytokines (IL-4, IL-5, IL-6 and IL-10) stimulate antibody production and promote mast cell and eosinophil proliferation[24,25]. The cytokine pattern secreted by T cells on viral antigen recognition is believed to exert a profound influence on both the type of disease caused by the infecting agent and the final outcome of the viral infection. In recent years, researchers have found that the prevalent Th1 pattern of secreted cytokines can be regarded not only as a mechanism contributing to inflammation and local tissue damage, but also as an appropriate response of the immune system to create conditions that hamper viral replication and eventually lead to HBV eradication[26]. Our experiment results showed that the effective ingredient of Sp could selectively increase the serum level of IL-2 (which is helpful for normal biologic immunity) and prevent the further increase of serum IL-6 (which can promote hepatocytes degeneration) in HBV-Tg (Table 3). These are important clues to therapeutic mechanism of Sp on chronic hepatitis B.
Then, what is the therapeutic mechanism of Sp It is still undefined. We suppose the explanations as follows: First, there are many chemical compositions such as peptide, alkaloids, saponin, macrolide, terpenoid in Sp. Coordinated action of them may be the main mechanism. Second, Sp is abundant in polypeptide and polysaccharide. Some compositions of them can stimulate cellular immunity, induce the differentiation of T lymphocyte and the generation of endogenous interferon, kill target cells and inhibit the replication of HBV. Third, mineral substances and vitamins in Sp also play main roles. Mineral substances are important in liver enzyme metabolism. Vitamins participate in multifold metabolism of liver. They can induce the generation of hepatocytes and enhance the detoxification function of liver.
In conclusion, the effective ingredient of Styela plicata has a definite effect on chronic hepatitis B. Our data provide valuable information for understanding the therapeutic role and the potential therapeutic mechanism of Styela plicata as an effective antiviral medicine in treating chronic hepatitis B.
The authors are grateful to Dr. Z Zeng (Department of Infectious Diseases, The First Teaching Hospital of Peking University) and Dr. AM Ji (Department of Pharmacy, Zhujiang Hospital, Southern Medical University) for their technical assistance.
| 1. | Liaw YF. Therapy of chronic hepatitis B: current challenges and opportunities. J Viral Hepat. 2002;9:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1714] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 3. | Akbar SM, Yamamoto K, Abe M, Ninomiya T, Tanimoto K, Masumoto T, Michitaka K, Horiike N, Onji M. Potent synergistic effect of sho-saiko-to, a herbal medicine, during vaccine therapy in a murine model of hepatitis B virus carrier. Eur J Clin Invest. 1999;29:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Tanikawa K. Recent advances in antiviral agents: antiviral drug discovery for hepatitis viruses. Curr Pharm Des. 2006;12:1371-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Leung N. Nucleoside analogues in the treatment of chronic hepatitis B. J Gastroenterol Hepatol. 2000;15 Suppl:E53-E60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 7. | Wang JH, Lu SN, Lee CM, Lee JF, Chou YP. Fatal hepatic failure after emergence of the hepatitis B virus mutant during lamivudine therapy in a patient with liver cirrhosis. Scand J Gastroenterol. 2002;37:366-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Raftos DA, Nair SV, Robbins J, Newton RA, Peters R. A complement component C3-like protein from the tunicate, Styela plicata. Dev Comp Immunol. 2002;26:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mayer AM, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer. 2003;105:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Mayer AM, Gustafson KR. Marine pharmacology in 2001-2: antitumour and cytotoxic compounds. Eur J Cancer. 2004;40:2676-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wan XX, Zhang X, Liu Q, Huang XP, Zeng FL, Wang SN. Inhibitory effect of ethanol extract from Styela plicata on HBsAg and HBeAg in vitro. Zhongguo Haiyang Yaowu. 2003;3:40-42. |
| 12. | Larkin J, Clayton M, Sun B, Perchonock CE, Morgan JL, Siracusa LD, Michaels FH, Feitelson MA. Hepatitis B virus transgenic mouse model of chronic liver disease. Nat Med. 1999;5:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Akbar SK, Onji M. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int J Exp Pathol. 1998;79:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Liu GZ, Jia YZ, Wang HM, Xiong YL. Observation on the stability of the replication and generation of HBV gene in transgenic mice. Shiyan Dongwu Kexue Yu Guanli. 2001;18:1-3. |
| 15. | Liu MC, Yu M, Zhang NL, Gong WB, Wang Y, Piao WH, Wang QH, Wang GQ. Dynamic analysis of hepatitis B virus DNA and its antigens in 2.2.15 cells. J Viral Hepat. 2004;11:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Guo WC, Mei B, Li YL, Zhang DF. The applied research on fluorogence probe PCR in diagnosing of HBV. Zhonghua Linchuang Yu Weisheng. 2004;3:305-307. |
| 17. | Zeng MD, WangTL , Wang BE. Consensus on evaluation of the diagnosis and efficacy of hepatic fibrosis. Zhenduanxue Lilun Yu Shijian. 2002;1:191-192. |
| 18. | Davies-Coleman MT, Beukes DR. Ten years of marine natural products research at Rhodes University. S Afr J Sci. 2004;100:539-544. |
| 19. | Fenical W, Jensen PR, Kauffman C, Mayhead SL, Faulkner DJ, Snicich C, Rao MR, Kantorowski EJ, West LM, Strangman WK. New Anticancer Drugs from Cultured and Collected Marine Organisms. Pharm Biol. 2003;41:6-14. [DOI] [Full Text] |
| 20. | Jimenez PC, Fortier SC, Lotufo TMC, Pessoa C, Moraes MEA, de Moraes MO, Costa-Lotufo LV. Biological activity in extracts of ascidians (Tunicata, Ascidiacea) from the northeastern Brazilian coast. J Exp Mar Biol Ecol. 2003;287:93-102. [DOI] [Full Text] |
| 21. | Wang R, Wan XX. The comparison of the inhibitory effect of ethanol extract from different species of ascidean on HBsAg and HBeAg in vitro. Zhongguo Yaolixue Tongbao. 2005;21:606-608. |
| 22. | Hu WJ, Wan XX. The screening of effective ingredient for anti-hepatitis B virus from Styela. Plicata. Zhongguo Yiyuan Yaofang Zazhi. 2004;24:202-203. |
| 23. | Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1341] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 25. | Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 591] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol. 2002;17:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH