Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3999
Revised: February 15, 2006
Accepted: February 28, 2006
Published online: July 7, 2006
AIM: To study the histological and pancreatitis-associated protein mRNA accumulation changes of pancreas from acute phase of caerulin-induced pancreatitis to recuperation in rats.
METHODS: Acute pancreatitis was induced by caerulein in male Wistar rats and followed up for 90 d by histological and mRNA analyses of pancreas. Pancreases were dissected at 0, 9, 24 h and 3, 5, 15, 30, 60, 90 d post-induction. Edema (E), polymorphonuclear neutrophil (PMN) infiltration, cytoplasmic vacuolization (V), zymogen granule depletion (ZD) and acinar disorganization (AD) were microscopically evaluated. Accumulation of pancreatitis-associated protein (PAP) and L13A mRNAs were quantified by real-time PCR.
RESULTS: The main histological changes appeared at 9 h post-induction for PMN infiltration and cytoplasmic V, while at 24 h and 3 d for E and ZD, respectively. All the parameters were recovered after 5 d, except for ZD which delayed more than 30 d. The main AD was observed after 15 d and values returned to normal after 30 d. Similarly to histological changes, accumulation of the PAP mRNA was increased at 9 h with the highest accumulation at 24 h and differences disappeared after 5 d.
CONCLUSION: From the acute phase to recuperation of pancreatitis, regeneration and re-differentiation of pancreas occur and PAP expression is exclusively an acute response of pancreatitis.
- Citation: Magaña-Gómez J, López-Cervantes G, Barca AMCL. Caerulin-induced pancreatitis in rats: Histological and genetic expression changes from acute phase to recuperation. World J Gastroenterol 2006; 12(25): 3999-4003
- URL: https://www.wjgnet.com/1007-9327/full/v12/i25/3999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.3999
Acute pancreatitis (AP) is an inflammatory disease of the pancreas associated with autodigestion of the gland, as a consequence of the intrapancreatic activation and releasing of digestive enzymes[1]. The pathogenic mechanisms involved in the development of acute pancreatitis are not well understood[2,3] although many studies on it are available. The acute period of pancreatitis is characterized by exocrine insufficiency as a result of important morphological alterations and changes on the expression of a number of genes in the pancreas[4,5]. If pancreatitis is not fatal, a stepwise regeneration of the morphology is followed[6].
Some events that regulate the severity of acute pancreatitis are known, involving common inflammatory and repair pathways[7,8]. Recently it was demonstrated that pancreatitis-associated protein (PAP), an acute phase protein, is itself an important determinant of disease severity[9]. The expression of this gene is low in the normal pancreas and becomes strongly augmentation after even mild pancreatic inflammation[10]. This augmentation is assumed in AP induced by caerulein, although it can be assayed in AP induced by retrograde injection of sodium taurocholate. Currently, pancreatitis is induced by supramaximal dose of caerulein and there is no report yet on histological and PAP mRNA changes in the pancreas as response of AP induction.
Therefore, the objective of this study was to evaluate the morphological and PAP mRNA changes in the acute and adaptive phases of pancreatitis induced by caerulein in rats. Morphological changes were evaluated by light microscopy and PAP mRNA levels by real-time PCR.
The experiments were carried out on 33 male Wistar rats (mean weight 100.8 g and 4-wk old). The animals were fed with standard laboratory chow and water ad libitum under controlled room conditions. Acute pancreatitis was induced according to the method of Dusseti et al[11]. The rats were injected intraperitoneally with caerulein (Sigma Chemical Co., St. Louis, MO, USA) at a dose of 50 μg/kg of body weight per hour for 6 h. The rats were sacrificed at h 9 and 24, and on d 3, 5, 15, 30, 60 and 90 respectively. Three control rats without caerulein injection were sacrificed at h 0 and on d 30 and 90 and dissected. Prior to sacrifice, the rats were anesthetized with 10-15 mg of tiletamine chlorohydrate and zolazepam chlorohydrate (Zoletil 50®) per kg of body weight. Pancreas was dissected for histological or RNA analysis. Feeding protocol and animal handling were approved by the local ethics committee.
Pancreatic sections were fixed in 10% formaldehyde, embedded in paraffin, cut (3-5 μm in thickness) with a semi-motorized rotary microtome (Leica RM-2145) and mounted onto slides. The sections were processed for H&E staining and examined by conventional light microscopy (Olympus microscope B × 50) with 20 ×, 40 × and 100 × objectives. Eight random images by objective were recorded by microscope video camera. A pathologist who was blinded to the treatment protocol scored the tissues according to Kyogoku et al[12] for zymogen granule depletion (0, absent; 1, less than 20%; 2, 20%-50%; 3, more than 50%), interstitial edema (0, absent; 1, expanded interlobular septa; 2, expanded intralobular septa; 3, separated individual acini), polymorphonuclear neutrophil (PMN) infiltration (0, absent; 1, less than 20 PMNs per intermediate power field (IPF); 2, 20-50 PMNs per IPF and 3, more than 50 PMNs per IPF), grade of vacuolization based on the percentage of acinar cells with cytoplasmic vacuoles per IPF (0, absent; 1, less than 20%; 2, 20%-50%; 3, more than 50%) and acinar disorganization based on the percentage of area without normal acinar distribution
(0, absent; 1, less than 20%; 2, 20%-50%; 3, more than 50%).
Extracted pancreas was immediately rinsed with RNase-free water (treated with 0.05% diethyl pyrocarbonate, DEPC), homogenized in 1 mL of TRIzol® reagent (GIBCO-BRL, Grand Island, NY, USA), frozen and stored at -70°C. Total RNA was extracted by phenol/chloroform extraction and isopropanol precipitation[13]. Accumulation of PAP mRNA (GenBank access No. NM_053289.1) was analyzed as indicator of pancreatic injury. L13A mRNA (GenBank access No. X68282) was also analyzed as housekeeping gene presumed to be invariant[14]. All the primers were designed with Primers3 software and synthesized by Sigma Genosys (Woodlands, TX, USA). Their sequences and PCR product sizes are listed in Table 1. Prior to the reverse transcription reaction, potentially contaminating residual genomic DNA was eliminated with DNase I (Invitrogen, Carlsbad, CA, USA) followed by reverse-transcribed using specific primers and SYBR Green RT-PCR reagents (PE Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Synthesized complementary DNA (cDNA) corresponding to 100 ng total RNA was used for real-time PCR.
| Primer | Sequence | GenBank No. | Product size (bp) |
| PAPfw | 5’ TGAATTATGTCAACTGGGAGAGG 3’ | NM-053289.1 | 318 |
| PAPrv | 5’ TTACTGCTTTCCAAGACATGAGG 3’ | ||
| rL13A/Fw | 5’ AAGCAGGTACTGCTGGG 3’ | X68282 | 261 |
| rL13A/Rv | 5’ CCAACACCTTGAGGCGTT 3’ |
Standards for the absolute quantification for PAP and L13A mRNAs were synthesized by Titan one Tube RT-PCR system (Roche Biochemicals, Indianapolis, IN, USA) with the same PCR parameters described above, according to the manufacturer’s instructions. Then, PCR products were purified with Wizard® SV gel and PCR clean-up system (Promega, Madison, WI, USA) and quantified by fluorometry.
Real-time quantification of PAP and L13A mRNAs was performed with an iCycler iQ detection system (BIO-RAD, Hercules, CA, USA). SYBR Green I assay was used for quantification of all specific genes. For PAP mRNA quantification of each 50 μL SYBR Green PCR, 9.0 μL cDNA (corresponding to 100 ng total RNA), 1.8 μL sense primer (10 μmol/L), 1.8 μL antisense primer (10 μmol/L), 25 μL SYBR Green PCR Master Mix (PE Applied Biosystems) and 12.4 μL PCR-grade water were mixed together. The PCR conditions were 1 cycle at 94°C for 4 min, then 35 cycles at 94°C for 30 s, at 61°C for 30 s and at 70°C for 30 s, with a single fluorescence measurement at the end of the 70°C for 30 s. For L13A mRNA quantification of each 50 μL SYBR Green PCR, 9.0 μL cDNA (corresponding to 100 ng total RNA), 1.2 μL sense primer (10 µmol/L), 1.2 μL anti-sense primer (10 μmol/L), 25 μL SYBR Green PCR Master Mix (PE Applied Biosystems) and 13.6 μL PCR-grade water were mixed together. The PCR conditions were 1 cycle at 94°C for 3 min, then 38 cycles at 94°C for 45 s, at 55°C for 45 s and at 72°C for 1 min, with a single fluorescence measurement at the end of the 72°C for 30 s. In both cases, a melting curve program (60-95°C with a heating rate of 0.5°C for 30 s and continuous fluorescence measurement) and a cooling step to 4°C were added.
Optical data obtained by real-time PCR were analyzed by using the default and variable parameters available in the software provided with the iCycler iQ optical system (BIO-RAD, Hercules, CA, USA). The PCR threshold cycle number (CT) for each cDNA standard and cDNA sample was calculated at the point where the fluorescence exceeded the threshold limit. The threshold limit was fixed along the linear logarithmic phase of the fluorescence curves at 10-20 SDs above the average background fluorescence. The number of amplicon cDNA copies was expressed relative to the amount of total cDNA present (ng). Although it might be difficult to determine the absolute amount of a cDNA present in different samples, quantification of test mRNA transcripts was normalized to a reference gene, the L13A gene.
Histological data were expressed as mean ± SE of the scores assigned by the pathologist and evaluated by ANOVA. Also, relation between PAP and L13A after absolute quantification was expressed as mean ± SE and compared using ANOVA test. P < 0.05 was considered statistically significant. NCSS 2001 statistical program was used.
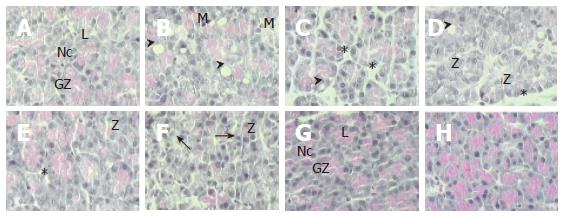
At the beginning (0 h), the architecture of pancreas was normal (Figure 1) with acinar cells exhibiting the typical epithelial polarity. Basal portion of the cells contained their nuclei and apical portion of the secretory vesicles and zymogen granules. Acinar cells in AP displayed moderate vacuolization and light inflammatory infiltration of neutrophils, with the highest score after 9 h post-induction. After 5 d, levels returned to the normal scores maintained to the end of the study. Edema was increased 9 h post-induction with the highest score at 24 h and returned to the normal level on d 5. Zymogen granule depletion was most pronounced on d 3 with later recovery. However, an additional depletion occurred on d 15, returned to the normal levels 30 d post-induction (Table 2). Although some degree of acinar disorganization was observed in the pancreas 9 h after induction, a significant increase was observed on d 15, and returned to the normal level 30 d post-induction (Table 2).
| Period | Zymogen granule depletion | Edema | PMN infiltration | Vacuolization | Acinar disorganization |
| 0 h | 0.25 ± 0.23a,b | 1.25 ± 0.31a,b | 0.00 ± 0.14a | 0.25 ± 0.26a | 0.5 ± 0.36a |
| 9 h | 1.00 ± 0.23b,c | 2.25 ± 0.31b,c | 0.75 ± 0.14b | 2.75 ± 0.26c | 0.75 ± 0.36a,b |
| 24 h | 1.40 ± 0.20c | 3.00 ± 0.28c | 0.60 ± 0.12b | 2.00 ± 0.24b | 1.00 ± 0.32a,b |
| 3 d | 3.00 ± 0.18e | 2.17 ± 0.25b,c | 0.67 ± 0.11b | 2.00 ± 0.22b | 1.33 ± 0.29a,b |
| 5 d | 2.00 ± 0.23c,d | 1.25 ± 0.31a,b | 0.00 ± 0.14a | 1.00 ± 0.26a,b | 0.75 ± 0.36a,b |
| 15 d | 2.57 ± 0.17d,e | 0.86 ± 0.23a | 0.00 ± 0.10a | 0.00 ± 0.20a | 2.14 ± 0.28b |
| 30 d | 1.60 ± 0.20c | 0.60 ± 0.83a | 0.00 ± 0.12a | 0.00 ± 0.24a | 0.80 ± 0.33a,b |
| 60 d | 0.00 ± 0.19a | 0.83 ± 0.25a | 0.00 ± 0.11a | 0.17 ± 0.22a | 0.66 ± 0.29a |
| 90 d | 0.25 ± 0.13a,b | 0.42 ± 0.18a | 0.00 ± 0.07a | 0.00 ± 0.15a | 0.92 ± 0.21a,b |
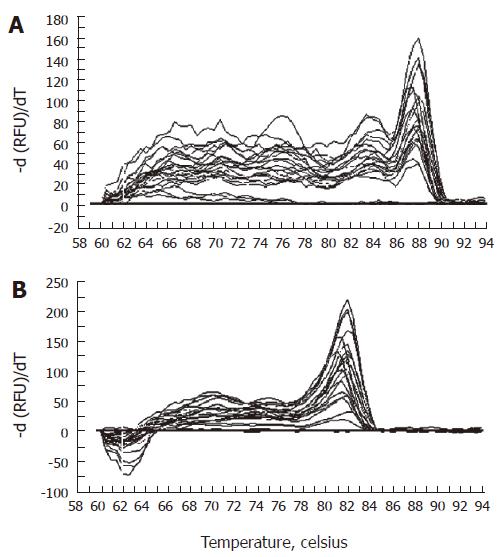
Melting curve analysis demonstrated that each of the primer pairs (Table 1) amplified a single predominant product with a distinct melting temperature (Tm) as shown for L13A and PAP cDNA in Figure 2. The Tm of products could be seen clearly as a peak in a first derivative plot. The rapid fall of Tm at 87.5°C and 82.0°C for L13A and PAP cDNA respectively indicated the presence of a specific product melting at this temperature.
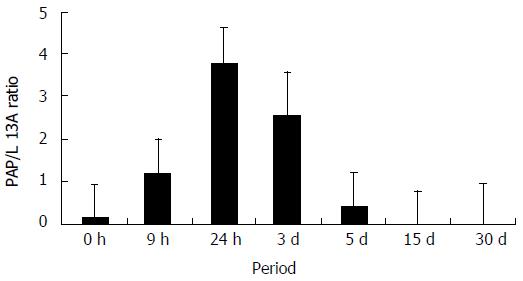
The absolute quantification was estimated from real-time PCR analyses using a standard curve of tenfold serial dilutions ranging from 1.78 × 109 copies to 1.78 × 105 copies of L13A mRNA and 1.46 × 109 copies to 1.46 ×105 copies of PAP mRNA, in 50 μL of volume reaction. As shown in Figure 3, the mRNA of PAP was detected in basal levels without caerulein (0 h). However, after induction of AP, the PAP mRNA expression was slightly augmented after 9 h, and 3.8-fold the basal level at 24 h. After 5 d, differences disappeared and the accumulation of PAP mRNA was decreased gradually to the basal level.
The acute phase of pancreatitis is characterized by a pattern of changes in the morphology of exocrine pancreas, expression of secretory proteins and mRNA levels of different genes[4,6,15,16]. The observed changes in our study resemble a mild form of AP, characterized by edematous fluid in the extra-cellular space, causing separation of lobules and acini[6]. Usually there is mild invasion by neutrophils, the acinar cells lose their zymogen granules, enlarging the acinar lumen. However, few studies[17-19] have analyzed the morphological changes after the acute phase of pancreatic response.
After acute response, re-differentiation of acinar cells and transition begin with regular acinar cell replete with dense zymogen granules beside cells with a smaller quantity of zymogen granules, on the same microscopic preparation. Intermediate cells change the typical architecture of the pancreas due to the loss of zymogen granules in a particular section. Additionally, the rough endoplasmic reticulum might be reduced and/or rearranged[6]. In this study, zymogen granule depletion and increase of acinar disorganization were presented after the acute phase of pancreatitis (determined by maximal PAP synthesis and its mRNA accumulation according to Iovanna et al[4] and our results, respectively). These changes are explained in the context of reconstitution of the normal morphology and function of the pancreas.
De Lisle and Logsdon[20] have shown the differentiation and re-differentiation of mouse pancreatic acinar cells. They immunolabeled acinar cells with monoclonal antibodies specific for acinar or duct cells and showed that more than 97% of the labeled area is acinar positive on d 3, which decreases to approximately 16% on d 9, and then returns to over 97% by day 21 of culture[20]. Lechene de la Porte et al[21] induced pancreatitis by the injection of trypsin in multiple sites of the rat pancreas and reported that pancreatic repair involves proliferation of cells from intact acini and tubular complexes. This process of differentiation and re-differentiation of pancreatic acinar cells explains our finding of the highest score for acinar disorganization on d 3 and 15 as well as complete recovering of zymogen granules 30 d post-induction.
In pancreatitis not only morphological changes were observed, but also molecular modifications occurred. Iovanna et al[4] showed that PAP is significantly increased 48 h after induction of pancreatitis by retrograde injection of sodium taurocholate in rats, compared to normal pancreas. In spite of methodological differences in the quantified PAP, our results agree with those of Iovanna
et al[4]. However, PAP mRNA accumulation is quite different between both studies. Differences could be attributable to the pancreatitis induction method, the severity of the problem, as well as the quantification method itself. In our study, after absolute quantification, values were normalized in respect to a housekeeping gene (L13A). Therefore, quantification by real-time PCR may better reflect the actual physiological changes.
Histology of pancreas showed a mild edematous pancreatitis induced by caerulein and the acute response was followed by recuperation process after 15 d of induction, according to acinar disorganization and zymogen granule depletion scores. Although PAP is related to the severity of pancreatitis, its function in vivo is not well established. Our findings support that PAP is a protein of acute response, because changes in levels are not observed in reconstitution of the pancreatic normal morphology. In addition, the PAP mRNA changes in AP induced by caerulein, measured by a most sensible technique of quantification as real-time PCR, are similar to those from AP induced by sodium taurocholate.
The authors are grateful to Dr. G Alpuche of the Instituto Potosino de Investigación Científica y Tecnológica, for allowing the use of the thermocycler for real time PCR analysis. The authors thank Adriana Bolaños for helping in manuscript preparation. Excellent technical assistance provided by René Valenzuela and Sofía López Valenzuela is greatly appreciated.
| 1. | Saluja AK. Pathophysiology of pancreatitis. Role of cytokines and other mediators of inflammation. Digestion. 1999;60 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 2. | de Dios I, Uruñuela A, Pinto RM, Orfao A, Manso MA. Cell-cycle distribution of pancreatic cells from rats with acute pancreatitis induced by bile-pancreatic obstruction. Cell Tissue Res. 2000;300:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci U S A. 2000;97:13126-13131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 277] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 4. | Iovanna JL, Keim V, Michel R, Dagorn JC. Pancreatic gene expression is altered during acute experimental pancreatitis in the rat. Am J Physiol. 1991;261:G485-G489. [PubMed] |
| 5. | Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457-G467. [PubMed] |
| 6. | Bockman DE. Morphology of the exocrine pancreas related to pancreatitis. Microsc Res Tech. 1997;37:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Nagar AB, Gorelick FS. Acute pancreatitis. Curr Opin Gastroenterol. 2004;20:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Weber CK, Adler G. Acute pancreatitis. Curr Opin Gastroenterol. 2003;19:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Zhang H, Kandil E, Lin YY, Levi G, Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol. 2004;39:870-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem. 1991;266:24664-24669. [PubMed] |
| 11. | Dusetti NJ, Tomasini R, Azizi A, Barthet M, Vaccaro MI, Fiedler F, Dagorn JC, Iovanna JL. Expression profiling in pancreas during the acute phase of pancreatitis using cDNA microarrays. Biochem Biophys Res Commun. 2000;277:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kyogoku T, Manabe T, Tobe T. Role of ischemia in acute pancreatitis. Hemorrhagic shock converts edematous pancreatitis to hemorrhagic pancreatitis in rats. Dig Dis Sci. 1992;37:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532-534, 536-537. [PubMed] |
| 14. | Jesnowski R, Backhaus C, Ringel J, Löhr M. Ribosomal highly basic 23-kDa protein as a reliable standard for gene expression analysis. Pancreatology. 2002;2:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Bödeker H, Fiedler F, Keim V, Dagorn JC, Iovanna JL. Pancreatitis-associated protein is upregulated in mouse pancreas during acute pancreatitis. Digestion. 1998;59:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Andrzejewska A, Dlugosz JW, Augustynowicz A. Effect of endothelin-1 receptor antagonists on histological and ultrastructural changes in the pancreas and trypsinogen activation in the early course of caerulein-induced acute pancreatitis in rats. World J Gastroenterol. 2005;11:1115-1121. [PubMed] |
| 17. | Elsässer HP, Adler G, Kern HF. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas. 1986;1:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Elsässer HP, Adler G, Kern HF. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas. 1989;4:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Gebhardt A, Ackermann W, Unver N, Elsässer HP. Expression of galectin-3 in the rat pancreas during regeneration following hormone-induced pancreatitis. Cell Tissue Res. 2004;315:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | De Lisle RC, Logsdon CD. Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol. 1990;51:64-75. [PubMed] |
| 21. | Lechene de la Porte P, Iovanna J, Odaira C, Choux R, Sarles H, Berger Z. Involvement of tubular complexes in pancreatic regeneration after acute necrohemorrhagic pancreatitis. Pancreas. 1991;6:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y