Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3933
Revised: July 15, 2005
Accepted: July 28, 2005
Published online: June 28, 2006
Although the association between inflammatory bowel disease and gastrointestinal infections has been suggested, the mechanisms involved in the pathogenesis of Crohn’s disease (CD) are still undetermined. We report the case of a man, who presented with mesenteric adenitis initially due to a Yersinia pseudotubercolosis infection, who was later diagnosed with Crohn’s disease. This case is in keeping with recent evidence in the literature which suggests that CD is a disease linked to abnormal immune responses to enteric bacteria in genetically susceptible individuals.
- Citation: Zippi M, Colaiacomo MC, Marcheggiano A, Pica R, Paoluzi P, Iaiani G, Caprilli R, Maccioni F. Mesenteric adenitis caused by Yersinia pseudotubercolosis in a patient subsequently diagnosed with Crohn’s disease of the terminal ileum. World J Gastroenterol 2006; 12(24): 3933-3935
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3933
Infection by Yersinia pseudotubercolosis is of increasing pathological importance. Yersinial subspecies should be considered as etiological agents in gatrointestinal infections. Patients with mesenteric adenitis normally present with symptoms similar to those of appendicitis. Such non complicated infections are, at present, successfully managed with systemic antimicrobial agents[1].
We report the case of a young man with terminal ileitis due to Yersinia pseudotubercolosis infection, in whom Crohn’s disease (CD) was subsequently diagnosed.
A 29 year old man was admitted to our GI unit with abdominal pain and diffuse arthralgies. He had demonstrated 5 kg weight loss in the last 7 months, which was probably due to a reduction in food intake because of abdominal symptoms. There was no family history of inflammatory bowel disease or cancer and no previous abdominal surgery. In the preceeding few months the patient had suffered from episodes of severe abdominal pain, emesis, fever (38-40°C), articular and back pain and demonstrated elevated inflammatory markers. The clinical condition of the patient remained poor despite antimicrobial treatment (ceftriaxone) and one month later he developed pleuropericardic effusion and active synovitis of the right knee.
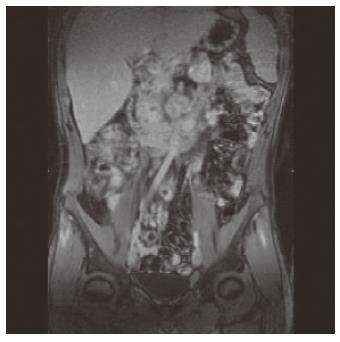
An ultrasound examination revealed thickening of the last 20 cm of the terminal ileum (5-6 mm) with light retro-dilatation and pathologically enlarged lymph nodes (1-2 cm). An abdominal MRI confirmed the wall thickening and the wall post iv contrast material enhancement, expression of active inflammation, and the presence of round and excavated mesenteric lymph nodes (Figure 1). Due to persistent abdominal pain, a laparoscopy was performed and biopsies of two mesenteric lymphnodes (transvers mesocolon) were collected. These revealed no histologic evidence of lymphoma. The diagnosis of Yersinia pseudotubercolosis infection was confirmed by serology and bacterial culture of the biopsied material. On the basis of suspected ileitis due to Yersinia pseudotubercolosis, cotrimoxazole and cefazime therapy was started. However, despite the antimicrobial treatment, intestinal and arthralgies were still present. Physical examination of the patient revealed poor condition. The abdomen was tender, with pain at palpation of the right iliac region. The tympanites was enhanced. Peristalsis was reduced with metallic sound.
There were no signs of immunodeficiency and most laboratory tests were normal, but indexes of inflammation were present (increased white blood cell count :14, 8/mm3; neutrophils 74%, ESR 60 mm/1 h; CRP 8,14 mg/dL) with associated anemia (HB 118 g/L; ferraemia 21,3 mcg/dL).
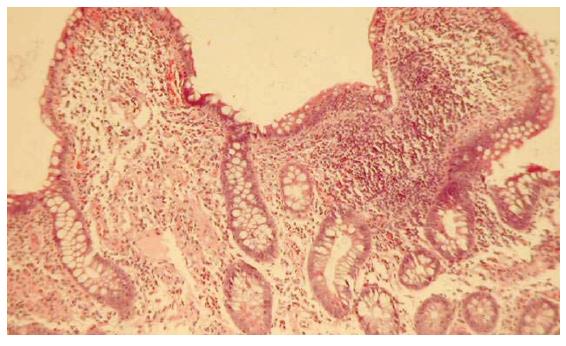
The presence of mesenteric lymphoadenitis and anemia suggested Coeliac disease as a possible diagnosis. However, upper gastrointestinal endoscopy and serological screening (EMA IgA, EMA IgG, antibodies anti-transglutaminase IgA) were all negative. Finally, a total colonoscopy with retrograde ileoscopy was performed. It showed erythema, edema, multiple erosions and ulcerations in the ileum. Random biopsies of the ileum were taken and the histopathological findings showed epithelial damage, distorsions of villous architecture and increased inflammatory infiltrate (lymphocytes and eosinophils) in the lamina propria and submucosa (Figure 2). These findings suggested a diagnosis of Crohn’s disease, treatment with oral mesalazine 2, 4 g/d was started and clinical improvement ensued. This improvement may also have related to a delayed effect of previous administration of systemic antibiotics. After one year of follow-up the patient was still in remission, and repeated endoscopy and ileal biopsies confirmed the diagnosis.
This case report describes the development of Crohn’s disease in a patient with a previous Yersinia pseudotubercolosis infection. In recent years, very little has been published on the relationship between Yersinia infections and Crohn’s disease[2,3], although microorganisms have long been suspected of causing CD. Yersinia pseudotubercolosis is a Gram-negative coccobacillus and a primary pathogen of wild and domestic animals and birds. It is an enteric pathogen that causes a variety of clinical symptoms in the human. These include diarrhoea, abdominal pain and post-bacteremia systemic symptoms such as fever, scarlattiniform skin rash, conjunctivitis, erythema nodosum and lymphadenophathy. Additional complications in the form of reactive arthritis, acute uveitis, coronary aneurysm and acute renal failure have also been reported[4].
When refrigerated, this organism survives while others do not. As a result, in a recent paper Hugot et al., have suggested the refrigerator as a new contributing factor in the development of CD[5]. In relation to the observation that CD is linked to a familial environmental risk factor related to modern lifestyle, the authors show temporal and geographical coincidences between the development of the refrigerator and the outbreak of CD.
This bacterium exhibits a marked tropism for lymphatic tissue and the characteristic histopathological findings in mesenteric lymph nodes suggest that the stimulation of T cells play a central role in the pathogenesis of this infection[6]. A key factor in the etiology of IBD is the chronic inflammatory process. Much evidence suggest that the transcription factor nuclear factor-kappa B (NFkB) plays an essential role in developing inflammation through the regulation of genes encoding pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes such as cyclooxigenase 2 (COX2) and inducible nitric oxide synthase (iNOS)[7]. Evidence has shown that Gram negative bacteria such as Yersinia pseudotubercolosis, target the eukaryotic signal transduction pathways that lead to NFkB activation[8]. NOD2 (renamed CARD15 by the International Nomenclature Committee) a gene involved in innate immunity, is an activator of the NFkB pathway[9]. The link between bacterial components, including lipopolysaccharide (LPS) and CD is strongly supported by the discovery that mutations in CARD15 predispose people to the disease[10]. Recent studies have demonstrated that a frameshift mutation in CARD15 may result in innate hyporesponsiveness to bacterial components, inducing an altered adaptive response to enteric bacteria[11].
This report supports previous evidence that CD is a disease of an abnormal immune response to enteric bacteria in genetically susceptible individuals. In common with previous studies associating infectious organism with CD, much work remains to elucidate whether the presence of the microorganisms is an epiphenomenon or actually a factor in the pathogenesis of CD.
| 1. | Butler T. Yersinia species including plague. Mandell, douglas and Bennett's principles and practice of infectious disease. Vol 5(2). Philadelphia: Churchill Livingstone 2000; 2406-2414. |
| 2. | Homewood R, Gibbons CP, Richards D, Lewis A, Duane PD, Griffiths AP. Ileitis due to Yersinia pseudotuberculosis in Crohn's disease. J Infect. 2003;47:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Lamps LW, Madhusudhan KT, Havens JM, Greenson JK, Bronner MP, Chiles MC, Dean PJ, Scott MA. Pathogenic Yersinia DNA is detected in bowel and mesenteric lymph nodes from patients with Crohn's disease. Am J Surg Pathol. 2003;27:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Abe J, Onimaru M, Matsumoto S, Noma S, Baba K, Ito Y, Kohsaka T, Takeda T. Clinical role for a superantigen in Yersinia pseudotuberculosis infection. J Clin Invest. 1997;99:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Hugot JP, Alberti C, Berrebi D, Bingen E, Cézard JP. Crohn's disease: the cold chain hypothesis. Lancet. 2003;362:2012-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | El-Maraghi NR, Mair NS. The histopathology of enteric infection with Yersinia pseudotuberculosis. Am J Clin Pathol. 1979;71:631-639. [PubMed] |
| 7. | Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 548] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Hugot JP. Inflammatory bowel disease: a complex group of genetic disorders. Best Pract Res Clin Gastroenterol. 2004;18:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3932] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 10. | Bonen DK, Ogura Y, Nicolae DL, Inohara N, Saab L, Tanabe T, Chen FF, Foster SJ, Duerr RH, Brant SR. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3498] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
S- Editor Wang J L- Editor Lalor PF E- Editor Zhang Y