Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3915
Revised: March 5, 2006
Accepted: March 13, 2006
Published online: June 28, 2006
AIM: To compare the one-day quadruple therapy with a standard 7-d triple therapy for H pylori eradication in a rural population of China.
METHODS: A total of 396 patients with 13C-urea breath test positive for H pylori were assigned into two groups: 239 patients received one-day quadruple therapy (amoxicillin 2000 mg qid; metronidazole 500 mg qid; bismuth citrate 900 mg qid and lansoprazole 60 mg once daily) and 157 patients received 7-d standard triple therapy (amoxicillin 1000 mg bid; clarithromycin 500 mg bid and lansoprazole 30 mg bid). All the patients underwent a 13C-UBT to assess the eradication of H pylori infection six weeks after treatment.
RESULTS: Two hundred and twenty-nine patients completed the one-day therapy (95.8%) and 148 patients completed the 7-d therapy (94.2%). The one-day therapy eradicated H pylori infection in 64 patients (27.95%). In contrast, 103 patients (69.59%) were H pylori negative after the 7-d therapy (P < 0.01).
CONCLUSION: This pilot study suggests there is no beneficial effect of the one-day therapy in treatment of H pylori infection compared with the 7-d standard therapy.
-
Citation: Zhang L, Shen L, Ma JL, Pan KF, Liu WD, Li J, Xiao SD, Lin SR, Classen M, You WC. Eradication of
H pylori infection in a rural population: One-day quadruple therapy versus 7-day triple therapy. World J Gastroenterol 2006; 12(24): 3915-3918 - URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3915.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3915
H pylori infection is the most common cause of gastritis, gastric ulcer, duodenal ulcer, and primary gastric MALT-lymphoma, and plays an important role in the development of gastric cancer[1-5].
Linqu County is a rural area with a high risk of gastric cancer in northern part of China, where 72% of adults and 52% of children aged 3-4 years are infected with H pylori[6]. Our previous studies carried out in this region suggested that H pylori infection is associated with an increased risk of gastric cancer[7,8] and a random trial in Linqu has demonstrated that one-time eradication of
H pylori infection can yieldcan a significant benefit on precancerous gastric lesions and a favorable trend on gastric cancer development [9].
Multiple therapies against H pylori infection are available at present with a higher cure rate than the single-antibiotic regimens. A triple-drug regimen, consisting of a proton-pump inhibitor, clarithromycin and amoxicillin for over a period of 7 d, has been recommended as a first-line therapy in H pylori management[10,11].
In 2003, Lara reported an efficacy of 95% with one-day quadruple therapy for the eradication of H pylori in comparison with 90% of 7-d triple therapy[12]. The advantages of the one-day therapy are low costs, low antibiotic resistance and a higher compliance, which motivated us to conduct a similar trial in Linqu County, Shandong Province, China. Herein, we report the results of a random trial of one-day quadruple therapy vs standard 7-d triple therapy in a rural population at high-risk of gastric cancer in China.
This was a prospective, open, standard controlled therapeutic trial. Subjects with positive 13C-urea breath test (13C-UBT) were allocated to receive one-day therapy and 7-d standard therapy respectively. The efficacy of the treatment was assessed six weeks after the therapy.
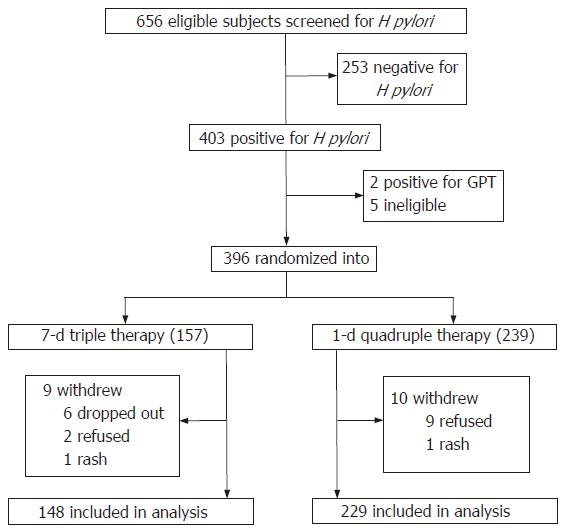
A total of 654 healthy residents aged 25-49 years from 3 villages were invited to participate in this study. All participants received a 13C-UBT to determine H pylori infection status. Trained field staff from the Peking University School of Oncology interviewed all the participants using a structured questionnaire containing age, sex, address, occupation, and previous histories of peptic ulcers, use of antibiotics and any allergic reaction to antibiotics. Exclusion criteria for the trial included negative H pylori infection, age younger than 25 years or older than 49 years, pregnant or breast-feeding women and people with allergic reaction or severe concomitant illness (e.g. cardiac, respiratory,renal, hepatic diseases and bleeding ulcer). Finally, 396 subjects from 3 villages qualified for entering this trial as illustrated in Figure 1. Among them, 239 patients from two villages (Xinzhuang and Zhujiagou) received a one-day quadruple therapy, and 157 patients from another village (Shiyazuei) received a 7-d triple therapy. The project was approved by the Institutional Review Board of the Peking University School of Oncology and an informed written consent was obtained from all the patients.
Details of the assay of 13C-UBT were described previously[13,14]. All participants were fasted overnight before baseline samples of exhaled CO2 were collected the following morning. Each patient was then requested to drink 20 mL of water with a pill of 80 mg 13C-urea (> 99%). Exhaled CO2 was collected in sampling tubes 30 min later. 13CO2 values were determined using a gas isotopic ratio mass spectrometer, and any concentration of 13CO2 at 30 min that exceeded the baseline concentration more than 4 parts per 1000 (> 0.4%) was regarded as a positive result. Samples of duplication were tested with an agreement of 97.2%. The sensitivity and specificity of the test were 94% and 83%, respectively[15].
All the 13C-UBT assays were blinded to the treatment aims. The end point of the study was the proportion of the patients with negative results of the 13C-UBT 6 wk after treatment in each aim.
In the one-day treatment group, a quadruple regimen consisted of amoxicillin 2000 mg qid; metronidazole 500 mg qid; bismuth citrate 900 mg qid and lansoprazole 60 mg once daily. And in the 7-d treatment group, the triple regimens consisted of amoxicillin 1000 mg bid, clarithromycin 500 mg bid and lansoprazole 30 mg bid.
To maintain a high compliance, all the patients in each village were organized into 4-5 groups and each group took medications in one household. The staff from the Peking University School of Oncology visited each household and monitored the medications for each patient at 8:00 and 20:00 everyday for the 7-d therapy and at 7:00, 13:00, 19:00 and 1:00 for the one-day therapy.
To monitor the adverse reaction related to the anti-H pylori therapies, each patient was interviewed using a questionnaire at six days after the treatment. Since metronidazole may injure the hepatic function, 2 mL blood was collected from each fasted patient before, 6 d and 6 wk after the treatment to test glutamic-pyruvic transaminase (GPT) in the laboratory of Linqu County Hospital.
For the efficacy analysis, the differences between the two eradication rates were assessed by the chi-square testing. A two-sided 95% confidence interval for the difference between success rates was calculated. All tests were two-sided and values of P < 0.05 were considered to indicate statistical significance.
Two hundred and twenty-nine patients (95.8%) completed the one-day therapy and 148 patients (94.3%) completed the 7-d therapy (Table 1). The treatment was unsuccessful for 10 patients in the one-day therapy group and 9 patients in the 7-d therapy group. In the one-day therapy group, 9 refused the treatment and 1 had to withdraw because of severe rash. Of the 9 patients in the 7-d therapy group, 2 refused the treatment, 6 dropped out and 1 had to withdraw because of severe rash also.
| Treatment | H pylori (+) | Age | Completed | Compliance (%) |
| 1-d | 239 | 39.3 ± 6.9 | 229 (M105, F124) | 95.8 |
| 7-d | 157 | 157± 7.3 | 148 (M78, F70) | 94.3 |
| Overall | 396 | 39.4 ± 7.1 | 377 (M183, F194) | 95.2 |
H pylori infection was eradicated in 64 of 229 patients (27.95%) in the one-day therapy compared with 103 of 148 patients (69.59%) in the 7-d therapy six weeks after the treatment (Table 2).
| Treatment | n | H pylori (-) | Eradication (95% CI) | P |
| 1-d | 229 | 64 | 27.95% (22.13-33.76) | < 0.01 |
| 7-d | 148 | 103 | 69.59% (62.18-77.00) |
As it shows in Table 3, the proportions of skin rash (5.4%) and abdominal bloating (12.8%) in the 7-d therapy group were significantly higher than that of the one-day therapy group (P = 0.013 and P = 0.035). In the one-day therapy group, the proportion of stool discoloration (33.2%) was significantly increased (P < 0.001).
| Treatment group | ||
| Side effects | 7-d n (%) | 1-d n(%) |
| Total | 148 | 229 |
| Rash | 8 (5.4)1 | 2 (0.01) |
| Diarrhea | 12 (8.1) | 10 (4.4) |
| Abdominal pain | 3 (2.0) | 10 (4.4) |
| Abdominal bloating | 19 (12.8)1 | 15 (6.6) |
| Stool discoloration | 14 (9.5) | 76 (33.2)1 |
| Headache | 5 (3.4) | 4 (1.75) |
| Dizziness | 4 (2.7) | 8 (3.5) |
| Nausea | 15 (10.1) | 17 (7.4) |
| Metallic taste | 0 | 4 (1.75) |
| Loss of appetite | 5 (3.4) | 7 (3.1) |
| Increase of appetite | 14 (9.5) | 13 (5.7) |
| Disappearance of abdominal pain | 1 (0.007) | 1 (0.004) |
| Stools becoming normal | 8 (5.4)1 | 3 (1.3) |
| Feeling better | 3 (2.0) | 17 (7.4)1 |
All 377 patients had a normal hepatic function before the treatment. Six days after the treatment, 91 patients (24.1%) showed abnormal GPT (> 40 IU), and in 9 patients (2.4%) GPT exceeded 100 IU, including 3 from the one-day therapy group and 6 from the 7-d therapy group. In the one-day therapy group, 26.6% of the patients (61/229) had a temporarily abnormal hepatic function (GPT > 40 IU) compared with 24.3% of the patients (36/148) in the 7-d therapy group. However, 36 d later, GPT level of most patients declined to below 40 IU.
The rates of H pylori eradication were 27.95% by one-day therapy and 69.59% by 7-d therapy in this pilot study. It was similar to one of our previous studies (64%)[9,16], in which 3411 patients received 14-d therapy with amoxicillin 1000 mg bid and omeprazole 20 mg bid. Wong et al reported 76.4% of eradication of H pylori by 7-d triple therapy in a trial in Changle County, China[17]. The result of the one-day anti-H pylori therapy in Linqu does not support Lara’s report[12]. The eradication rate of H pylori is lower (27.95%) than that reported by Lara (95%).
In our study, quality control was enforced throughout the trial. The patients in the one-day therapy group were confined to the village throughout the medication day and monitored by our staff according to the schedule. Although a few patients were out of the village during the 7 d treatment, they were allowed to take the medicine during away and required to contact our staff by cell phone immediately after medicine was taken on schedule.
The reason for the poor eradication rate of H pylori in one-day therapy is unclear. The antibiotics are widely used in China and all over the world. As a result, antibiotic resistance is a growing and severe problem worldwide. Kato et al reported the prevalence of H pylori resistance to clarithromycin, metronidazole and amoxicillin is 29%, 24% and 0% (51 of H pylori isolated strains) among Japanese children[18]. Kim et al studied 652 H pylori isolated strains from Korea residents, and the resistance rates were 40.6% (metronidazole), 5.9% (clarithromycin), 5.3% (tetracycline), and the resistance to metronidazole increased from 1994 to 1999 (from 33.3 to 47.7%)[19]. Li reported the H pylori resistance to metronidazole in Chinese is between 28% and 80%, and to clarithromycin is < 5%, respectively[20]. Although the relatively high-dosages of antibiotics are used in the one-day therapy, it is worth investigating the time frame associated with the effectiveness of proton-pump inhibitors and antibiotics. If the proton-pump inhibitor plays a more effective role in the middle or late stage of the treatment in combination with antibiotics, one-day therapy may result in low efficacy in eradication of H pylori. In general, the efficiency of H pylori eradication in general population is lower than that in clinical patients. The clinical patients with active inflammation seem to be more sensitive to anti-H pylori therapy than those with long-term infection and with severe precancerous lesions.
In conclusion, our trial confirms a high efficacy rate of eradication of H pylori infection by a standard 7-d triple therapy; whereas, one-day quadruple therapy with high dosage of amoxicillin, metronidazole, bismuth citrate and lansoprazole produces a low eradication rate of H pylori. Because the efficacy of H pylori eradication reported by Lara et al can not be confirmed, the one-day quadruple therapy could not be recommended to use in a large scale study in future.
| 1. | International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter pylori. Lyon, France: IARC Press 1994; 61. |
| 2. | Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35 Suppl 12:90-97. [PubMed] |
| 4. | Correa P. Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiol Biomarkers Prev. 1996;5:477-481. [PubMed] |
| 5. | An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 745] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Blot WJ, You WC, Chang YS, Kneller RW, Jin ML, Li JY, Zhao L, Liu WD, Zhang JS. Helicobacter pylori antibodies in relation to precancerous gastric lesions in a high-risk Chinese population. Cancer Epidemiol Biomarkers Prev. 1996;5:627-630. [PubMed] |
| 7. | You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Ma JL, You WC, Gail MH, Zhang L, Blot WJ, Chang YS, Jiang J, Liu WD, Hu YR, Brown LM. Helicobacter pylori infection and mode of transmission in a population at high risk of stomach cancer. Int J Epidemiol. 1998;27:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Gail MH, You WC, Chang YS, Zhang L, Blot WJ, Brown LM, Groves FD, Heinrich JP, Hu J, Jin ML. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Control Clin Trials. 1998;19:352-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Walsh JH, Peterson WL. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 253] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Malfertheiner P, Mégraud F, O'Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Lara LF, Cisneros G, Gurney M, Van Ness M, Jarjoura D, Moauro B, Polen A, Rutecki G, Whittier F. One-day quadruple therapy compared with 7-day triple therapy for Helicobacter pylori infection. Arch Intern Med. 2003;163:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | You WC, Zhang L, Gail MH, Ma JL, Chang YS, Blot WJ, Li JY, Zhao CL, Liu WD, Li HQ. Helicobacter pylori infection, garlic intake and precancerous lesions in a Chinese population at low risk of gastric cancer. Int J Epidemiol. 1998;27:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Klein PD, Malaty HM, Martin RF, Graham KS, Genta RM, Graham DY. Noninvasive detection of Helicobacter pylori infection in clinical practice: the 13C urea breath test. Am J Gastroenterol. 1996;91:690-694. [PubMed] |
| 15. | Zhang L, Jiang J, Chang YS, Zhao L, Liu WD, Ma JL, Pan KF, Zhang JS, Blaser MJ, Perez-perez GI. Diagnosis of H pylori infection in a population with a high risk of stomach cancer by 13C-UBT and ELISA. Chin J Oncol. 1995;17:131-132. |
| 16. | You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, Ma JL, Pan KF, Liu WD, Hu Y. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1051] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 18. | Kato S, Fujimura S, Udagawa H, Shimizu T, Maisawa S, Ozawa K, Iinuma K. Antibiotic resistance of Helicobacter pylori strains in Japanese children. J Clin Microbiol. 2002;40:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, Graham DY, Kwon DH. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Li YY, Sha WH. Treatment of Helicobactor pylori infection: analysis of Chinese clinical trials. World J Gastroenterol. 2000;6:324-325. [PubMed] |
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L