INTRODUCTION
The intestinal mucosa is an important route of entry for microbial pathogens. The single layer of enteric epithelial cells that lines the intestinal mucosa is the initial site of interaction between the host and entero-invasive microbial pathogens. Following bacterial entry, intestinal epithelial cells rapidly initiate the innate and acquired immune response. Within the first few hours after bacterial invasion, the intestinal mucosa produces mediators that orchestrate the onset of an early inflammatory response. Characteristics of this program include the increased production and release of chemokine[1], cytokines[2,3] and nitric oxide (NO)[4]. These molecules can act as early signals to activate an acute mucosal inflammatory response and enhance the ability of epithelial cells to produce cytokines that regulate mucosal immune responses.
NO is generated by the conversion of L-arginine to L-citrulline by NO synthase (NOS), which exists in three isoforms, encoded by a separate gene. The expression of inducible NOS (iNOS, encoded by NOS2, one of the isoforms) is regulated in various cell types and can be upregulated by stimulation with several cytokines or with bacterial lipopolysaccharide (LPS). iNOS can also be expressed constitutively, in some specialized cells, such as human lung epithelial cells[5]. In human colon epithelial cell lines, the iNOS expression and production of NO are upregulated following stimulation with a combination of interferon-γ (IFN-γ) and interleukin-1 (IL-1) or tumor necrosis factor-α (TNF-α)[6]. NO can mediate a wide array of physiological effects in the intestine that are relevant to neuronal regulation of vascular function, and epithelial barrier integrity[7]. Moreover, NO produced by intestinal epithelial cells could play a protective role in the host response to enteric microbial pathogens[8,9]. Entero-invasive bacteria directly activate expression of iNOS and NO production in intestinal epithelial cells[10]. The NO in the intestine has been implicated in the suppression of microbial entry into epithelial cells by increasing the protective barrier through the release of epithelial mucus[11] and the induction of intestinal fluid secretion[12]. In addition to direct innate immune action, NO has immuno-regulatory effects relevant to the control of infection on modulating the subsequent acquired immune response[13].
Interleukin 6 (IL-6) is a pleiotropic cytokine that is produced by many different cell types and mediates several physiological responses, such as humoral immune response, acute-phase reactions, and hematopoiesis. In the intestine, IL-6 enhances IgA production in murine Peyer’s patch B cells[14] and human appendix B cells that express the IL-6 receptor[15]. Intestinal epithelial cells may also secrete IL-6 and TGF-β to enhance IgA secretion and suppress IgM production by mucosal B cells, whose function is highly dependent upon local production of T cell cytokines[16,17]. These facts indicate that IL-6 plays an important role in the mucosal immune response.
We have previously established a coculture system of human colonic epithelial cells (Caco-2) with murine lymphocytes of Peyer’s patch and demonstrated that the cell-cell contact between epithelial and immune cells is important in modulating the epithelial barrier and transport function in response to challenge of Shigella LPS[18]. Although accumulating evidence suggests that the interaction or “cross-talk” between epithelial cells and lymphocytes of the intestine is crucial in the immune response to bacterial invasion, no report has clearly demonstrated how the interaction between epithelial cells and lymphocytes, particularly from the Peyer’s patch, affects the release of important immuno-regulatory mediators, such as NO and IL-6, at rest and in response to bacterial infection. The present study was undertaken using the established coculture system of Caco-2 epithelial cells with lymphocytes of Peyer’s patch to investigate NO and IL-6 release in response to Shigella LPS challenge. We also cocultured Caco-2 epithelial cells with lymphocytes from iNOS knockout mice to investigate the involvement of NO in regulation of IL-6 release in response to Shigella LPS.
MATERIALS AND METHODS
Isolation of Peyer’s patch lymphocytes
Wild-type (C57) mice and iNOS knockout mice of C57 background (SPF, 6-8 wk-old) were obtained from the Animal House of Chinese University of Hong Kong. The lymphoid follicles of the mouse Peyer’s patch were excised from the intestinal serosal side and placed in 10 mL PBS, supplemented with 20 mL/L FBS (Invitrogen Co., Grand Island, NY) and 2% penicillin-streptomycin (Invitrogen Co.). The collected patches were triturated by pipetting up and down a few times and smashing through a metallic grid (mesh: 100). Individual lymphocytes were released in the medium below the metallic grid. The lymphocytes were washed with PBS, in which the distribution of Peyer’s patch T and B cell populations was consistent with previous data when they were checked by flow cytometry[19], and diluted to a concentration of 1 × 107 cells/mL prior to mixed culture with Caco-2.
Enteric epithelial cell culture
Human colonic cell line Caco-2 was purchased from American Type Culture Collection (Rockville, MD). The cells were grown in Dulbecco modified Eagle’s minimal essential medium (DMEM; Invitrogen Co.) with 100 mL/L FBS, 2 mmol/L L-glutamine (Invitrogen Co.), 100 μmL/L non-essential amino acid (Invitrogen Co.), 200 units/mL penicillin and 200 μg/ml streptomycin, at 37°C, 50 mL/L CO2. To maintain the growth of an uniform polarized epithelial monolayer, Caco-2 cells were seeded at a density of 3 × 105 cells on a floating permeable support, which was made of a membrane filter (Millipore, 0.45 μm pore size) with a silicone rubber ring attached on top of it for confining the cells (0.45 cm2 growth area).
Coculture configurations
Three types of culture configurations were established in this study: (1) Caco-2 culture alone: Caco-2 cells were cultured as a homogenous polarized monolayer according to the epithelial cell culture method mentioned above and they served as the epithelial cell control; (2) Coculture: Caco-2 cells (3 × 105) completely mixed with Peyer’s patch lymphocytes (1 × 106), and then were seeded on a permeable support described above and maintained up to the 5th d at 37°C in a 50 mL/L CO2 atmosphere; (3) Lymphocytes of Peyer’s patch alone: lymphocytes were cultured in an equal volume of the coculture (1 × 106/well in 96-well plate) at 37°C in a 50 mL/L CO2 atmosphere as the lymphocyte control.
Shigella F2a-12 LPS pretreatment
When the cells cultured on the membrane filter reached confluence on the 4th d, Shigella F2a-12 LPS (5 μg/mL, obtained from the Immunology Department of Institute of Microbiology & Epidemiology, Academy of Military Medical Sciences) was added on the apical side of the epithelium monolayer growing on the membrane filters and treated for 24 h at 37°C in a 50 mL/L CO2 atmosphere.
Detection of NO release (Griess colorimetric assay)
After confluent Caco-2 monolayers on the Millipore filter were challenged by Shigella F2a-12 LPS pretreatment for 24 h, cell culture supernatants were collected and kept at -20°C until evaluation of NO level in the medium by Griess reagent system (Promega, USA). Briefly, nitrite accumulation was determined by mixing equal volumes of cell culture medium and the Griess reagent (10 mL/L sulfanilamide in 50 mL/L phosphoric acid, and 1 mL/L N-1-napthylenediamine dihydrochloride in water). The absorbance (A520-A550) was read on a 96-well microplate reader. Standard curves were constructed with known concentrations of sodium nitrite. The values for the amount of NO were converted from the absorbance values based on the standard curve constructed for each assay.
Enzyme-linked immunosorbent assay (ELISA)
After confluent Caco-2 monolayers on the Millipore filter were challenged by Shigella F2a-12 LPS pretreatment for 24 h, the culture supernatants were collected and kept at -20°C until evaluation of mIL-6 level in the culture medium. The IL-6 was measured using a mouse IL-6 quantitative colorimetric sandwich ELISA from Biosource (USA) according to the manufacturer’s instructions. The values were calculated on the basis of a standard curve constructed for each assay.
Statistical analysis
All data are expressed as means ± SE. The number of experiments represents independent measurements on separate monolayers. Statistical analysis was performed by repeated measure analysis of variance (ANOVA), and “P” values less than 0.05 were considered statistically significant.
RESULTS
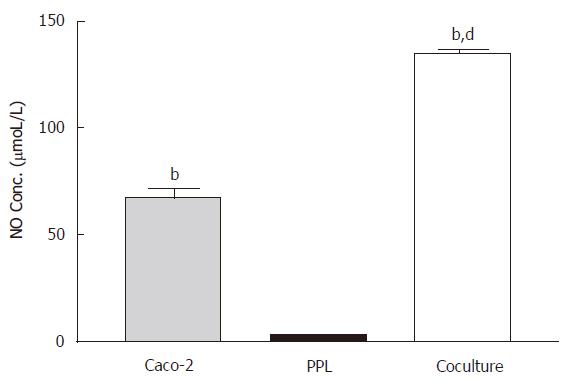
Constitutive release of NO from Caco-2 epithelial cells was potentiated by the cocultured lymphocytes of Peyer’s patch
In the absence of LPS stimulation, measurements of NO from the culture medium showed a moderate level of NO release from Caco-2 cells (67.64 ± 13.79 μmoL/L) but almost negligible amount (3.15 ± 0.46 μmol/L) in lymphocytes of Peyer’s patch from wild-type mice; however coculture of both produced over 2-fold (P < 0.001) increase in NO release (Figure 1).
Figure 1 Enhancement of the constitutive NO release in the Caco-2 cocultured with lymphocytes of Peyer’s patch.
The level of NO in different culture conditions (Caco-2 alone: gray bar; PPL alone: black bar; coculture: white bar) was measured by Griess reagent system and the bar values were derived based on a standard curve. The values represent the means ± SE for 6-12 different samples; bP < 0.001 vs PPL; dP < 0.001 vs Caco-2.
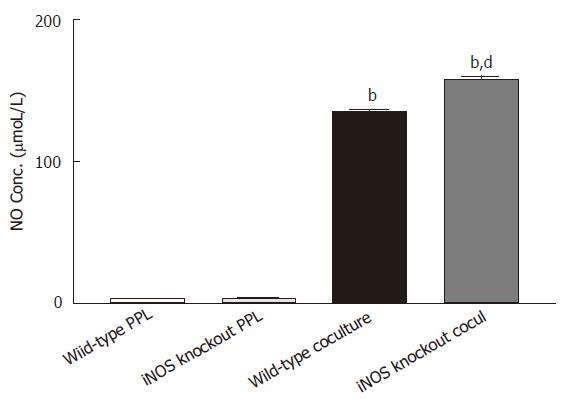
The increase in NO production in the coculture could be due to either potentiation of epithelial release by the cocultured lymphocytes or induction of NO release from lymphocytes by coculturing with epithelial cells. To distinguish this, lymphocytes from iNOS knockout mice were used. If coculture induced NO production in lymphocytes, this would not be observed with iNOS knockout lymphocytes. However, as shown in Figure 2, high level of NO (158.29 ± 2.62 μmoL/L) was still observed in the coculture of Caco-2 with iNOS knockout lymphocytes, confirming the epithelial origin of NO. Moreover, coculure with iNOS knockout lymphocytes produced a level of NO significantly greater than that produced by coculture with lymphocytes of intact iNOS (P < 0.001), indicating an inhibitory role of iNOS from lymphocytes in regulating NO release from epithelial cells.
Figure 2 Release of NO is upregulated to the epithelial source Caco-2 cocultured with PPL from iNOS knockout mice.
Two sets of cocultures were established (Caco-2 cocultured with PPL of wild-type mice; and Caco-2 cocultured with PPL of iNOS knockout mice). The data are indicated by the following bars (white bar: PPL only from wild-type control mice; light gray bar: PPL only from iNOS knockout mice; black bar: coculture of Caco-2 and PPL from wild-type control mice; dark gray bar: coculture of Caco-2 and PPL from iNOS knockout mice). The level of NO production was measured by Griess colorimetric assay and the bar values were derived based on a standard curve. The values present the means ± SE for 6-8 different samples; bP < 0.001 vs its own PPL; dP < 0.001 vs wild-type coculture.
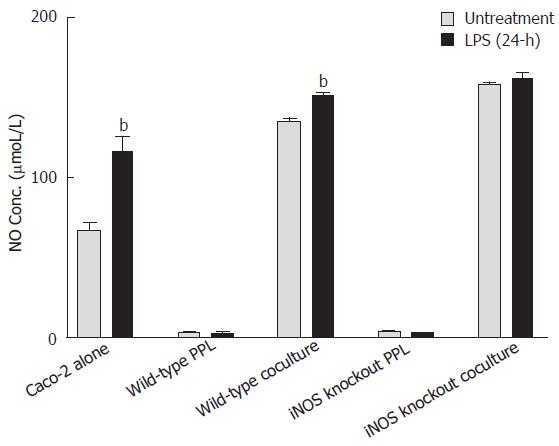
Shigella LPS induced NO release from Caco-2 epithelial cells but with little effect on either lymphocytes of Peyer’s patch or the coculture with lymphocytes of iNOS knockout mice
As shown in Figure 3, the level of NO in the culture medium of Caco-2 alone and the coculture with lymphocytes of wild-type mice was significantly increased 24 h after LPS treatment as compared to its untreated control (from 67.64 ± 13.80 μmol/L to 116.44 ± 34.57 μmol/L, P < 0.001; from 135 ± 4.4 μmol/L to 151.35 ± 4.84 μmoL/L, P < 0.001); however, no changes in NO levels were observed in the lymphocytes after LPS treatment (from 3.15 ± 0.46 μmol/L to 2.46 ± 0.52 μmol/L, P > 0.05). LPS treatment for 24 h did not significantly produce further increase in the already-high levels of NO release from the coculture of epithelial cells with lymphocytes from iNOS knockout mice (Figure 3).
Figure 3 Comparison of NO release induced by Shigella LPS in various Caco-2 and PPL culture configurations.
Shigella LPS was added to five different cultures (set 1: Caco-2 alone; set 2: wild-type PPL only; set 3: Caco-2 and wild-type PPL coculture; set 4: iNOS knockout PPL alone; set 5: Caco-2 and iNOS knockout PPL coculture) and the level of NO release was measured by Griess colorimetric assay and derived based on a standard curve. The black bar and the gray bar represent 24 h treatment with and without LPS, respectively. The values represent the means ± SE for 6-14 different samples. Statistical significance relative to its own un-treatment group was indicated by bP < 0.001.
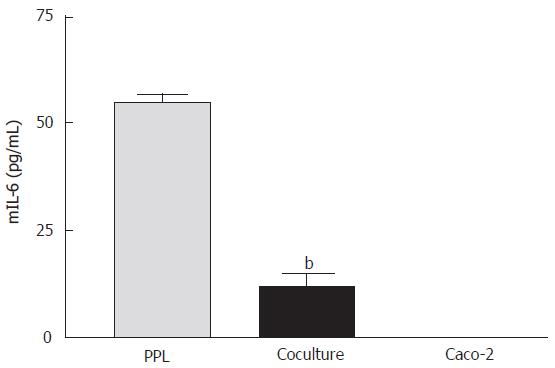
Suppression of mIL-6 release from lymphocytes of Peyer’s patch by cocultured epithelial cells
The release of mIL-6 from different cultures was measured using a mouse ELISA kit. The results showed that mIL-6 release was detected from the lymphocytes of Peyer’s patch alone (55.23 ± 2.98 pg/mL) but not from Caco-2 cells, indicating no cross-reactivity of the mouse antibody with human IL-6. When the lymphocytes were cocultured with Caco-2 cells, mIL-6 release was significantly reduced (Figure 4).
Figure 4 Suppression of mIL-6 release from PPL by cocultured Caco-2 epithelial cells.
The bars (gray: wild-type PPL only; black: coculture of Caco-2 and PPL; white: Caco-2 only control) indicate the level of mIL-6 release 4 d after culture. The level of mIL-6 was measured by mIL-6 ELISA and the values were derived based on a standard curve. The values represent means ± SE; n = 4; bP < 0.001 vs PPL.
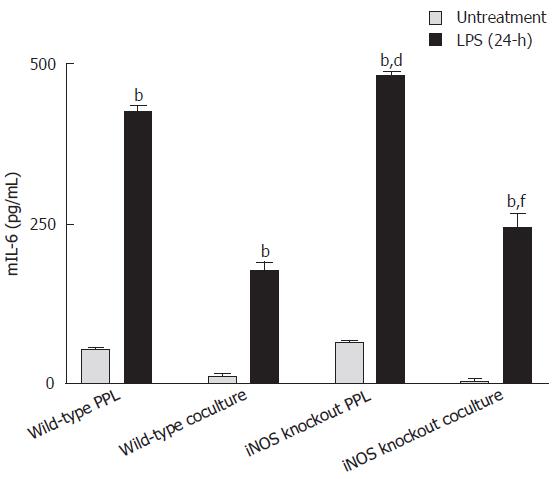
LPS triggered mIL-6 release from both lymphocytes of Peyer’s patch and cocultures, which was further enhanced with iNOS knockout lymphocytes
As shown in Figure 5, mIL-6 levels in either lymphocytes alone or in cocultured with Caco-2 cells were significantly increased 24 h after Shigella F2a-12 LPS treatment. Furthermore, the levels of LPS-induced increases in mIL-6 release were significantly higher in groups with lymphocytes from iNOS knockout mice (Figure 5), indicating an inhibitory role of iNOS from lymphocytes in regulating mIL-6 release.
Figure 5 Comparison of mIL-6 release induced by Shigella LPS in various Caco-2 and PPL culture configurations.
Shigella LPS was added to four different cultures (set 1: wild-type PPL only; set 2: Caco-2 and wild-type PPL coculture; set 3: iNOS knockout PPL only; set 4: Caco-2 and iNOS knockout PPL coculture) and the level of mIL-6 release was measured by mIL-6 ELISA and the values were derived based on a standard curve. The black bar and the gray bar represent 24 h treatment with and without LPS, respectively. The values represent the mean ± SE for 4 different samples; bP < 0.001 vs its own untreatment group, dP < 0.001 vs wild-type PPL 24-h after challenge Shigella LPS and fP < 0.001 vs wild-type coculture 24-h after Shigella LPS challenge.
DISCUSSION
Kerneis S and colleagues[19] have demonstrated that murine Peyer’s patch lymphocytes cocultured with human differentiated Caco-2 cell monolayers are able to induce a phenotypic conversion of enterocytes into cells sharing structural and functional properties with M cells. This phenomenon reveals the profound influence of Peyer’s patch lymphocytes on intestinal epithelial phenotypes[20,21]. And our previous study indicated that Peyer’s patch lymphocytes may modulate intestinal epithelial barrier and ion transport function in homeostasis and host defense via cell-cell contact and cytokine signaling[18]. The present study has further demonstrated the importance of the interaction between intestinal epithelial cells and lymphocytes of Peyer’s patch in regulating the release of NO and IL-6 from Caco-2 epithelial cells and lymphocytes of Peyer’s patch, respectively, at rest and in response to Shigella LPS challenge.
The present study has demonstrated constitutive release of NO from enteric epithelial cells but not from lymphocytes of Peyer’s patch. We also firstly found that the cocultured Peyer’s patch lymphocytes potentiated NO release from the enteric epithelial cells. It is known that the constitutive and iNOS activity has the highest level in intestinal villus cells, which is resembled by Caco-2 cells, and the lowest is in crypt cells[22]. Further studies have also demonstrated that the enterocytes regulate their own ion transport processes, either in basal condition or in the presence of active secretion, through the activation of a constitutive NOS-NO pathway, functioning as a braking force of cAMP-induced ion secretion[23]. It has also been reported that iNOS-derived NO is a key mediator of early villous re-epithelialization following acute mucosal injury[24]. These facts indicate that constitutive NO production may have a physiological role in intestinal epithelial cells. It has been proposed that NO synthesis in intestinal epithelial cells is up-regulated through some molecules or cytokines including T-lymphocytes derived cytokines[25] during cell-cell contact with lymphocytes[26]. The observed potentiation of NO release from epithelial cells by Peyer’s patch lymphocytes is consistent with the above notion. Our data also suggest that NO from the lymphocytes, although almost negligible, may be involved in negatively regulating NO release from enteric epithelial cells since lymphocytes from iNOS knockout mice further enhance NO release from the epithelial cells. This also rules out the possibility of coculture-induced NO release from the lymphocytes, confirming epithelial release of NO potentiated by the lymphocytes.
Upon Shigella F2a-12 LPS challenge, the present results showed that NO from epithelial cell Caco-2 significantly increased but not from lymphocytes of Peyer’s patch alone groups. Witthoft[10] has indicated that entero-invasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. NO is involved in the protective mechanisms in the gastrointestinal tract. Enterocyte-derived NO changes intestinal monolayer permeability and ion channel via iNOS and COX-2[27-30]. In the acute phase response, shigellosis inflammation is characterized by increased cell turnover in the lamina propria (LP) and the epithelium, increased iNOS expression in the surface epithelium, and apoptosis, which seems to be associated with LP macrophages[31]. However, NO production in the coculture with C57 wild type lymphocytes further up-regulated by the LPS treatment, yet there was almost no increase in NO production in the coculture with iNOS knockout lymphocytes following LPS challenge. This observation suggests that the coculture of Caco2 and iNOS knockout lymphocytes has become insensitive to the LPS challenge. The iNOS knockout lymphocytes might lack the additional factors that are crucial for LPS-induced NO release. This is a likely scenario since recent studies demonstrated that host defense function of NO in intestinal mucosal immune response is regulated by IL-12 and/or IFN-γ[32], which are deficient in the iNOS knockout mice[33].
Another interesting finding from the present study is that epithelial cells could also suppress mIL-6 secretion by lymphocytes of Peyer’s patch with cell-cell contact. The mIL-6 level in both coculture groups was significantly reduced as compared to that of lymphocytes of Peyer’s patch alone. It is clear that IL-6 is produced by a number of cell types including antigen-presenting cells, lymphocytes and even epithelial cells via stimulating of endotoxin and a number of other stimuli (TGF-β, IL-1β, etc). However, the mIL-6 in the coculture system is only detectable from mouse lymphocytes of Peyer’s patch in our previous study[18]. It should be noted that there is evidence that epithelial cells could inhibit IL-6 secretion from lymphocytes by the interaction between epithelial cells and lymphocytes of Peyer’s patch in homeostasis. After shigella LPS treatment for 24 h, mIL-6 level was obviously increased in all groups. More interestingly, the LPS-induced mIL-6 release was significantly greater in groups with lymphocytes from iNOS knockout mice, indicating an inhibitory role of iNOS in regulating mIL-6 release from lymphocytes their own. Mucosal production of IL-6 is important because this cytokine may regulate a number of local and systemic immune responses, including IgA secretion[16], macrophage differentiation, T cell proliferation, and acute phage protein synthesis[34] in liver and intestine as well as active transcription of a variety of cytokines and receptors[35]. Accumulating evidence[36] suggests that NO inhibits the LPS-induced IL-6 production and this effect may be a direct effect or caused by inhibited PGE2 production, or both PGE2 and NO may act in an autocrine or in a paracrine manner. NO from epithelial cells is easy to reconcile NF-γB activity with the needs of host defense through autocrine pathway to produce a rapid response to a pathogenic stimulus that is shut down promptly so as to minimize host damage[37]. This study only investigated NO release and mIL-6 secretion from different sources to illustrate the cross-interaction between epithelial cells and lymphocytes in homeostasis and host defense. Further studies are required to identify their regulatory mechanism of NO from intestinal epithelial cells to reduce LPS-induced IL-6 production of Peyer’s patch lymphocytes.
In summary, the cross-interaction between intestinal epithelial cells and lymphocytes of Peyer’s patch regulates host defense mechanism. Lymphocytes of Peyer’s patch can induce a basal constitutive production of NO from epithelial cells. This basal level NO may play an important physiological role in the enteric epithelium in the absence of infection. During infection, NO release from epithelial cells is further upregulated. Conversely, epithelial cells can downregulate IL-6 secretion of Peyer’s patch lymphocytes, while LPS can upregulate IL-6 release. Furthermore, NO from lymphocytes appears to play an inhibitory role in the epithelial NO release and their own IL-6 release in response to LPS. Taken together, NO and IL-6 are important immuno-regulatory mediators in response to bacterial infection through the interaction between epithelial cells and Peyer’s patch lymphocytes.