INTRODUCTION
Interleukin-2 (IL-2) is a 15 kD cytokine synthesized and secreted primarily by activated T cells[1,2]. IL-2 plays a central role in the augmentation of cell-mediated immune responses by stimulating and supporting a broad range of immune cells, including T and B cells, macrophages, and natural killer (NK) cells[3]. Furthermore, IL-2 can also stimulate the production of lymphokine-activated killer (LAK) cells, which are derived from NK cells and can effectively mediate antibody-dependent cellular toxicity. The administration of recombinant human IL-2 (rhIL-2), in combination with LAK cells, has been shown to be of value in the treatment of patients with advanced melanoma or renal cell carcinoma[4]. However, the in vivo efficacy of rhIL-2 treatment has been limited by its associated severe systemic toxicity and difficulties in maintaining prolonged high concentration of the cytokine in the tumor microenvironment, which is likely necessary to induce local anti-tumor immune responses[5,6]. To circumvent these problems, several approaches to selectively target IL-2 to tumor sites have been employed, particularly the use of immunoconjugates[7-9].
Murine monoclonal antibodies (mAbs) have been extensively used as carriers to target therapeutic agents to tumor sites for diagnostic and therapeutic modalities over the past decade. Despite some highly encouraging diagnostic data obtained with this approach, the general therapeutic efficacy has been rather disappointing[10-13]. Several major obstacles related to the mAb approach have been identified, including relatively long half-life of the immunocomplex, human anti-mouse antibody (HAMA) response and inability of the immunoconjugate to penetrate large tumor masses[14,15]. To date, several approaches of engineering conventional murine mAbs have been developed for more effective cancer targeting therapy. One approach is the development of Fv portion of an antibody consisting of the VH and VL domains. This version of antibody is the smallest antibody fragment to bear the antigen binding site. Furthermore, the reports showed that a genetically engineered single-chain Fv(sFv) with binding activity could be produced by connecting the carboxyl terminus of one V domain to the amino terminus of the other with a flexible peptide linker[16]. Advantages of this small antibody fragment include improved clearance of immunocomplex from the circulation, better penetration in solid tumors and lower immunogenicity[17-20]. Other approaches include the development of recombinant immunotoxins and antibody-immunostimulatory molecule conjugates[21-24].
In this study we constructed genetically a recombinant bispecific fusion protein, sFv/IL-2 consisting of sFv and IL-2 portions. We hoped that this protein could target IL-2 to tumor sites to overcome those obstacles mentioned above. The kind of recombinant proteins could be generated in bacteria. But protein from bacteria is not active and must be solubilized, oxidized, and renatured in vitro. Even so it is impossible to avoid the loss of its bioactivities. A different approach to produce active fusion protein would be to use the more sophisticated refolding machinery that is located in the endoplasmic reticulum (ER) of mammalian cells. But, fusion proteins were usually expressed at very low level in mammalian cells thus it was not efficient for large-scale preparation[25-27]. For these reasons, we tried to find out an efficient mammalian expression system for the large-scale preparation of the recombinant humanized sFv antibody/hIL- 2 fusion protein[28], and an effective method for preparing the therapeutic doses of the fusion protein.
MATERIALS AND METHODS
Construction of expression plasmids
Mammalian expression plasmids encoding the humanized sFv antibody/IL-2 fusion proteins were constructed. Baculovirus expression plasmid pAcHCs-520C9sFv containing a humanized 816-bp 520C9 sFv cDNA fragment, and the plasmids pLW46 and plasmid pLW42 containing either wild type or mutant human IL-2 cDNA fragments, respectively, were kindly provided by Chiron Corporation (Emeryville, CA). The mutant human IL-2 cDNA contains a single base substitution at position 173 (G→C) which converts amino acid Cys 58 of the wild type protein to Ser, thus eliminating a disulphide bond critical to the biological function of IL-2. Primers used for the amplification of 520C9 sFv and human IL-2 cDNA fragments from the above plasmids were synthesized at the Institute for Molecular Biology (MOBIX), McMaster University (Hamilton, ON). The forward primer AB9883 (5’-CTT AAG CTT GCC ACC ATG GAC ATG AGG GTC CCC GCT-3’) was used to amplify the sFv cDNA containing a Kozak consensus sequence prior to the initiating ATG and a terminal HindIII restriction site. The reverse primer AB7824 (5’-CC GAA TTC TTT AAT CTC CAG TTT TGT CCC TTG GGC-3’) contained an EcoRI site 3’ to the sFv cDNA fragment. The 440-bp mutant human IL-2 and wild type human IL-2 cDNA fragments derived from pLW42 and pLW46 were subjected to PCR amplification using the forward primer AB7822 (5’-GGG GAA TTC GGT GGC GGT GGC TCG GGC GGT GGT GGG TCG GGT GGC GGC GGA TCT ATG CCT ACT TCA AGT TCT ACA AAG AAA ACA CAG-3’), which contained a terminal EcoRI site and a coding sequence for a 15 amino acid, glycine rich linker peptide (NH2-GGGGSGGGGSGGGGS-COOH) 5’ to the human IL-2 cDNA fragment. The reverse primer AB4749 (5’-GAC CTC GAG TCA GTG TTG AGA TGA T-3’) contained a terminal XhoI site adjacent to the termination codon of the human IL-2 cDNA fragment. PCR products were purified from 0.8% agarose gels and ligated into T-ended pBluescript using T4 DNA ligase (Gibco/BRL, Burlington, ON). The ligation mixture was then used to transform competent DH5α cells (Gibco/BRL). Plasmids with inserts were digested with HindIII and EcoRI for the sFv, or EcoRI and XhoI for the human IL-2 cDNA fragments. These cDNA fragments were purified from agarose gels using QIAGEN Gel purification kit (Qiagen, Chatsworth, CA) and ligated into the HindIII/XhoI site of the mammalian expression vector pcDNA3.1(+) (Invitrogen, San Diego, CA) to produce the recombinant plasmids pcDNA-H520C9sFv-hIL-2 and pcDNA-H520C9sFv-mhIL-2. Fidelity of the PCR-amplified constructs was confirmed by fluorescence-based double-stranded DNA sequencing (MOBIX). The ligation mixture was then used to transform competent DH5α cells. Transformants containing both the humanized 520C9 sFv and IL-2 cDNA fragments were confirmed by restriction enzyme analysis. The constructs were subsequently purified using QIAGEN plasmid Midi kits and dissolved in Tris-EDTA buffer (pH 7.4) to a concentration of 0.4 mg/mL.
Establishment of stable cell lines
The 293 cells used for stable expression of the cDNAs encoding H520C9sFv-hIL-2 or H520C9sFv-mhIL-2 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco/BRL) containing 10% fetal calf serum (FCS; HyClone Laboratories, Logan, UT) at 37°C in a 50 mL/L CO2 incubator. Cells grown to 20% confluency were transfected with 5 μg of the plasmid constructs using 30 μL of SuperFect transfection reagent (Qiagen) as described by the manufacturer. As a negative control, pcDNA3.1 (+) was used to transfect 293 cells under the same conditions. Stable transfectants were selected in DMEM containing 10% FCS and 600 μg/mL G418 (Gibco/BRL) for two weeks, after which the concentration of G418 was increased to 1.2 mg/mL. Clones secreting the H520C9sFv-hIL-2 fusion protein were identified by ELISA using either a mouse anti-human IL-2 mAb MAB202 (R&D Systems, Minneapolis, MN) or a rabbit anti-human IL-2 polyclonal antibody EP100 (Genzyme, Cambridge, MA). In brief, 100 μL of supernatant from different clones was added to a 96-well plate coated with MAB202 (1:250 dilution), and incubated at 37°C for 2 h. After three washes with PBST, the plate was incubated with EP100 (1:200 dilution) at 37°C for 1 h, washed and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polyclonal antibody (Gibco/BRL) (1:1000 dilution) for 1 h at 37°C. After three additional washes with PBST, colour development was performed with peroxidase substrate (Bio-Rad, Hercules, CA) and the resulting OD determined at 405 nm in a microplate reader (Bio-Tek Instrument Inc., Winooski, VT). Clones producing the highest amount of fusion protein were selected and grown in DMEM containing 10% FCS for 72 h. The conditioned medium was collected for subsequent precipitation and analysis.
Determination of concentration of fusion protein
The quantitative indirect ELISA was performed to determine the concentration of fusion protein of conditioned medium. A 96-well microtiter plate was coated with MAB202 (1:250 dilution), and incubated at 37°C for 2 h overnight at 4°C. The 100 μL of serial diluted standard rhIL-2 and diluted conditioned medium were then added to each well, and incubated at room temperature for 2 h. After three washes with PBST, the plate was incubated with EP100 (1:200 dilution) at 37°C for 1 h, washed and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polyclonal antibody (Gibco/BRL) (1:1000 dilution) for 1 h at 37°C. After three additional washes with PBST, colour development was performed with peroxidase substrate and the resulting OD determined at 405 nm in a microplate reader as above.
Immunoprecipitation and purification of fusion proteins
H520C9sFv-hIL-2 and H520C9sFv-mhIL-2 fusion proteins were immunoprecipitated using MAB202 coupled with CNBr-activated Sepharose CL-4B (Pharmacia Biotech, Baie d’Urf, QC). Twenty-five microliters of a 1:1 (v/v) slurry of the absorbent suspended in 20 mmol/L Tris-HCl buffer (pH 7.8) were added to 1.5 mL of the conditioned medium with gentle shaking at 4°C for 4 h. The Sepharose beads were washed three times using Tris-HCl buffer, then 50 μL of 1 × SDS-PAGE sample buffer containing β-mercaptoethanol was added to the beads. After heating at 100°C for 3 min, the beads were pelleted by centrifugation and the supernatant collected for immunoblot analysis.
For affinity purification of the fusion protein, the conditioned medium was applied to an MAB202-Sepharose CL-4B affinity column at a flow rate of 5 column volume per h. The column was then washed three times with 20 mmol/L Tris-HCl buffer (pH 7.8) and the fusion protein was eluted with Pierce Gentle Ag/Ab Elution Buffer (Rockford, IL) as described by the manufacturer.
Immunoblot analysis
Twenty microliters of the above supernants were separated on a 15% SDS-polyacrylamide gel under reducing conditions. Proteins were electroblotted onto nitrocellulose membranes (Bio-Rad, Richmond, CA) and the efficiency of protein transfer was determined by staining the blots with Ponceau S (Sigma, St. Louis, MO). Membranes were washed briefly in TBST to remove the Ponceau S stain, blocked in TBST containing 5% skimmed milk at 4°C overnight and incubated at room temperature for 2 h with EP100 (1:1000 dilution). Following three 20 min washes in TBST, the membranes were incubated for 2 h with an HRP-conjugated goat anti-rabbit IgG polyclonal antibody (1:2000 dilution). After washing in TBST, membranes were developed with the Renaissance Chemiluminescence Reagent kit (DuPont, Boston, MA) as described by the manufacturer.
IL-2 bioactivity assay
Biological activity of the fusion proteins was determined by standard IL-2-dependent CTLL-2 cell proliferation[2] and cytotoxicity assays[29]. CTLL-2 cells deprived of rhIL-2 (Chiron, Emeryville, CA) for 3 d were seeded into a 96-well plate at 1 × 104 cells per well and incubated with either serially diluted rhIL-2 standard or fusion protein at 37°C in a 50 mL/L CO2 incubator. After 24 h, 1 μCi of [3H]-thymidine (DuPont, Boston, MA) was added to each well and the cells incubated for an additional 12 h. Cells were harvested onto glass fibre filters (Whittaker Corp., Walkersville, MD), dried and the incorporated radioactivity was counted in a Beckman scintillation counter (Fullerton, CA).
For bioactivity neutralization experiments, 2 ng/mL of rhIL-2 and the same concentration of IL-2 in H520C9sFv-hIL-2 were incubated with increasing concentrations of the anti-rhIL-2 neutralizing mAb MAB202 for 2 h at 37°C in a 96-well microtiter plate. As a control, mouse IgG was added to H520C9sFv-hIL-2 instead of MAB202. Following this incubation period, 1 × 104 CTLL-2 cells were added to each well. The assay mixture, in a total volume of 100 μL/well, was incubated for 24 h at 37°C in a 50 mL/L incubator and pulsed with 1 μCi/well [3H]-thymidine for the final 12 h. The cells were then harvested onto glass fiber filters and [3H]-thymidine incorporation was determined.
In the cytotoxicity assay, peripheral blood mononuclear cells (PBMCs) from healthy individuals were separated on a Histopaque 1077 density gradient (Sigma, St. Louis, MO). The PBMCs were cultured in RPMI 1640 medium (Gibco/BRL) supplemented with 10% FCS containing either 1000 IU/mL rhIL-2 or same dose of the H520sFv-hIL-2 fusion protein as determined by the above CTLL-2 assay for 3 d to produce LAK cells. Cytotoxicity of the resulting LAK cells was assessed by a 4 h Calcein AM (Molecular Probe, Eugene, OR) release assay with NK-resistant Daudi cells (ATCC, Rockville, MD) used as target cells. Each well of a 96-well plate contained 1 × 104 Daudi cells which had been pre-labelled with fluorescent Calcein AM. To achieve different effector/target cell ratios, an increasing number of LAK cells was added to each well. The total volume of culture medium per well was 0.1 mL. Following co-incubation of LAK and Daudi cells at 37°C for 4 h, the cells were pelleted and the fluorescent intensity value (Valuesample) of 50 μL of cell supernatant from each well containing both types of cells was measured with a Cytofluor 2300 fluorescence reader system (Millipore, Bedford, MA). The maximal release value (Valuemaximal) of the fluorescent tag was determined by adding an equal volume of 1% (v/v) Triton X-100 to the wells with only target cells. The spontaneous release value (Valuespontaneous) of the tag was given by the fluorescent intensity of 50 μL of cell supernatant from the wells without any LAK cells. The percentage of specific lysis for each sample of LAK cells was calculated by the formula (Valuesample-Valuespontaneous)/(Valuemaximal-Valuespontaneous) × 100.
Determination and specificity of antigen-binding activity of H520C9sFv-hIL-2
Antigen-binding activity was measured by indirect ELISA using cultured SKOV3ip1. Human ovarian carcinoma SKOV3ip1 cells which express high levels of p185[30] were maintained in DMEM containing 10% FCS and used for determination of antigen-binding activity of the H520C9sFv-hIL-2 fusion protein. Cells (1 × 104) were added to individual wells of a 96-well microtiter plate one day before the assay. Serially diluted samples of the H520C9sFv-hIL-2 or a chemically-conjugated control mAb 520C9-rhIL-2 were added to each well of the plate and incubated at 37°C for 2 h[31]. After three washes with PBST, EP100 (1:250 dilution) was added to the cells and incubated for 2 h. Following three washes with PBST, HRP-conjugated goat anti-rabbit IgG polyclonal antibodies (Gibco/BRL) (1:1000 dilution) were added to the cells. After 2 h incubation, the cells were washed three times with PBST, colour was developed with the addition of peroxidase substrate (Bio-Rad, Hercules, CA) and the OD of the solution was determined at 405 nm in a microplate reader as described.
To demonstrate specificity for p185 binding, SKOV 3ip1 cells (1 × 104) grown in 96-well plates were treated with either serially diluted intact 520C9 mAb or normal mouse IgG (0.001 to 10 nmol/L) at 37°C for 2 h prior to the addition of 10 nmol/L H520C9sFv-hIL-2 fusion protein. Indirect ELISA was then performed as described above.
Statistical analysis
Data are presented as mean ± SD. Statistical differences between groups were determined by using Student’s t-test. For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Determination of concentration of the fusion protein
The concentration of conditioned media from 293 cells stably transfected with either pcDNA-H520C9sFv-hIL-2 or pcDNA-H520C9sFv-mhIL-2 were at 102.0 ± 4.2 or 101.0 ± 5.6 mg/L, respectively.
Detection of the IL-2 moiety in the fusion protein
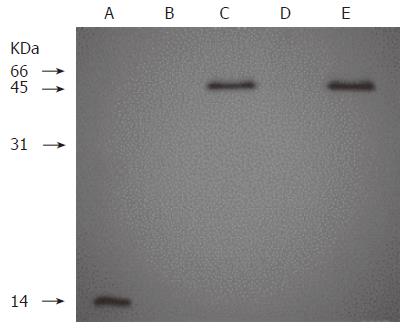
To confirm the presence of the IL-2 moiety in the fusion protein, the MAB202 immunoprecipitates of conditioned media from 293 cells stably transfected with either pcDNA3.1(+), pcDNA-H520C9sFv-hIL-2 or pcDNA-H520C9sFv-mhIL-2 were analysed by Western blotting using EP100. As shown in Figure 1, a single band of 45 kD was observed in the conditioned media from 293 cells transfected with either pcDNA-H520C9sFv-hIL-2 (lane C), or pcDNA-H520C9sFv-mhIL-2 (lane E), but not in the culture supernatant of 293 cells (lane B) or the conditioned medium from 293 cells transfected with pcDNA3.1(+) (lane D). Furthermore, the migration positions of these fusion proteins on SDS-PAGE were consistent with their predicted molecular mass. As a positive control, EP100 also recognized rhIL-2 (lane A).
Figure 1 Western blot analysis of recombinant humanized sFv antibody/IL-2 fusion proteins stably expressed in 293 cells.
Lane A: 0.5 μg of rhIL 2; Lanes B, C, D and E: The supernants from conditioned medium of 293 cells, 293 cells transfected with pcDNA-H520C9sFv-hIL-2, pcDNA3.1 (+) and pcDNA-H520C9sFv-mhIL-2, respectively. Samples were separated on a 15% SDS-polyacrylamide gel under reducing conditions, transferred to nitrocellulose membranes and immunoblotted using the anti-human IL-2 polyclonal antibody, EP100. The fusion proteins, H520C9sFv-hIL-2 and H520C9sFv-mhIL-2 were shown to migrate as single bands of 45 kD.
Cell proliferation and cytotoxicity assays
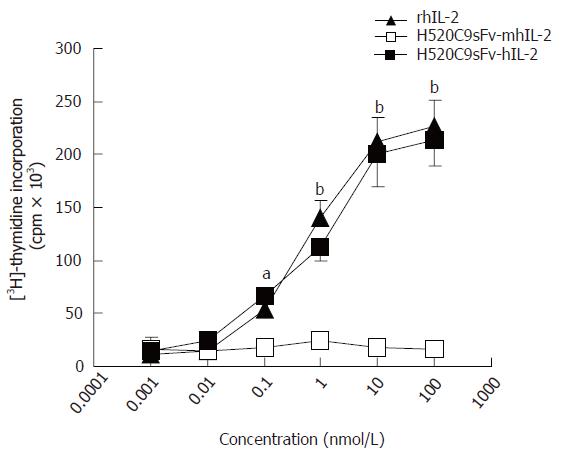
The activity of the H520C9sFv-hIL-2 fusion protein to support the proliferation of IL-2-dependent CTLL-2 cells and to generate LAK cells was compared to those of rhIL-2. As shown in Figure 2, conditioned medium from 293 cells transfected with pcDNA-H520C9sFv-hIL-2, but not pcDNA-H520C9sFv-mhIL2, possessed similar IL-2 bioactivity to rhIL-2 standard. These results indicated that the bioactivity of the IL-2 moiety in terms of its ability to support the growth of CTLL-2 cells was maintained in the H520C9sFv-hIL-2 fusion protein.
Figure 2 IL 2 activity of recombinant humanized sFv antibody/IL-2 fusion proteins.
IL 2 activity of rhIL-2, or conditioned medium from 293 cells transfected with either pcDNA-H520C9sFv hIL 2 or pcDNA-H520C9sFv mhIL 2 was measured by [3H] thymidine incorporation in CTLL 2 cells. The data are the mean ± SD from three separate experiments. bP < 0.01 for rhIL-2 vs H520C9sFv mhIL 2, and P > 0.05 vs H520C9sFv hIL 2; aP < 0.05 for rhIL-2 vs H520C9sFv mhIL 2, and P > 0.05 vs H520C9sFv hIL 2.
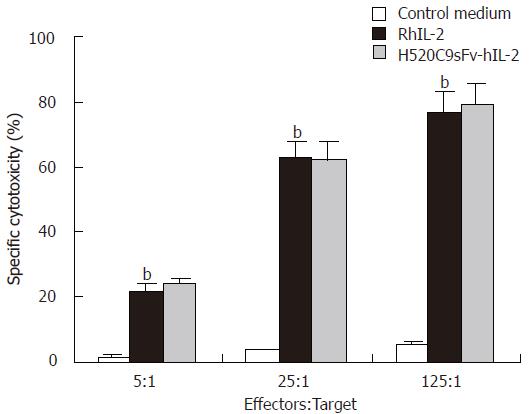
In the cytotoxicity assay directed against target Daudi cells, LAK cells generated by 1000 IU/mL of IL-2 of the H520C9sFv-hIL-2 fusion protein, as determined by the above CTLL-2 assay, demonstrated similar effect in the killing of Daudi cells compared to LAK cells activated by 1000 IU/ml of rhIL-2 (Figure 3). The specific lyses of Daudi cells by LAK cells stimulated by the H520C9sFv-hIL-2 and rhIL-2 were 79% and 77%, respectively, at an effector : target ratio of 125:1. No cytotoxic effect was observed when conditioned medium from control 293 cells transfected with pcDNA3.1(+) was used.
Figure 3 LAK cell-mediated cytotoxicity induced by H520C9sFv-hIL-2 fusion protein.
Human LAK cells (effector) were generated by incubation of fresh PBMCs with either conditioned medium from control 293 cells transfected with pcDNA 3.1 (+), rhIL 2 or equivalent dose of H520C9sFv hIL 2. Lysis of Daudi cells (target) was determined using a 4 h Calcein AM release assay. The data are the mean ± SD from three separate experiments. bP < 0.01 for rhIL-2 vs control medium, and P > 0.05 vs H520C9sFv hIL 2.
Neutralization of IL-2 activity in H520C9sFv-hIL-2
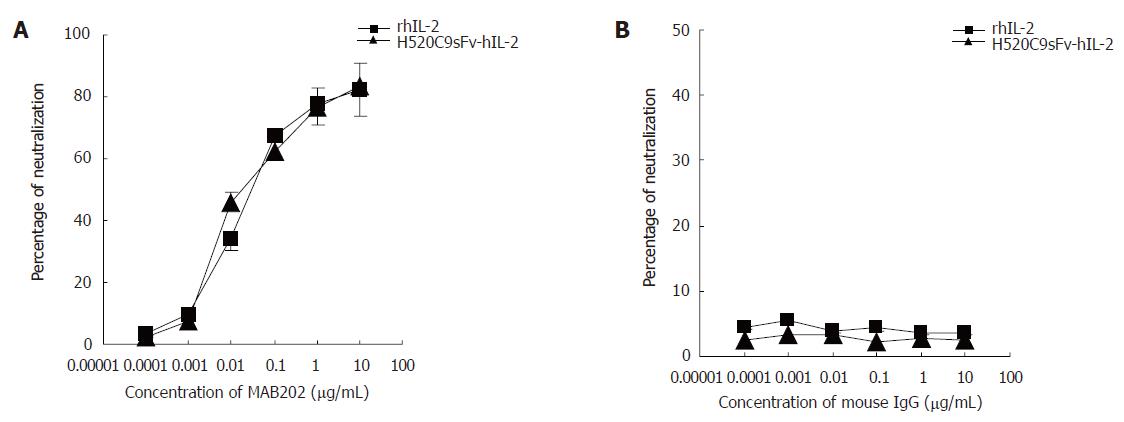
To determine if the proliferation effects of the H520C9sFv-hIL-2 fusion protein were strictly IL-2-dependent, inhibition studies using IL-2 neutralizing antibodies were performed. As shown in Figure 4A, the proliferative effects of both rhIL-2 and H520C9sFv-hIL-2 on CTLL-2 cells, as measured by [3H]-thymidine incorporation of the CTLL-2 cells were inhibited by MAB202. Approximately 50% inhibition of [3H]-thymidine incorporation for 2 ng/mL rhIL-2 or same dose of H520C9sFv-hIL-2 was achieved at an MAB202 concentration of approximately 0.0176 and 0.022 μg/mL, respectively. No inhibitory effect was observed when control mouse IgG was added (Figure 4B).
Figure 4 Inhibition of stimulatory effects of rhIL 2 or H520C9sFv hIL 2 on CTLL 2 cells by the anti human IL 2 neutralizing antibody, MAB202.
Based on the molecular mass of either IL-2 or H520C9sFv-hIL-2 fusion protein, the neutralization dose 50 for 2 ng/ml rhIL 2 or the same dose of H520C9sFv hIL 2 was determined to be approximately 0.0176 and 0.022 μg/mL MAB202, respectively (A). No inhibitory effect was observed using control mouse IgG (B). The data are the mean ± SD from three separate experiments (B). A: P > 0.05 for rhIL-2 vs H520C9sFv hIL 2; B: P > 0.05 for rhIL-2 vs H520C9sFv hIL 2.
Antigen-binding assays
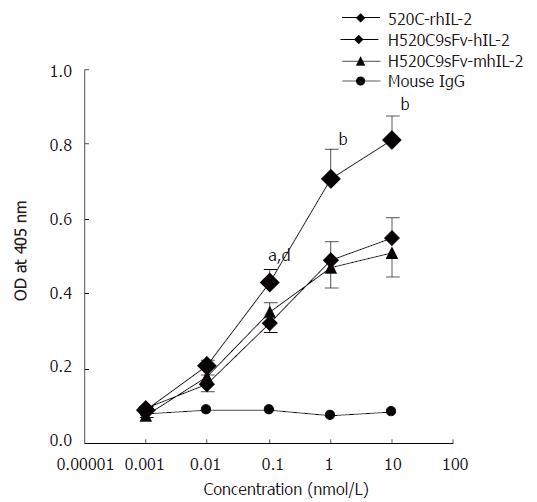
The antigen-binding specificity of the H520C9sFv-hIL-2 fusion protein was determined by indirect ELISA using SKOV 3ip1 cells, which overexpressed p185. As shown in Figure 5, the conditioned medium from 293 cells transfected with either pcDNA-H520C9sFv-hIL-2 or pcDNA-H520C9sFv-mhIL-2 displayed binding activity for p185 on the cell surface of SKOV 3ip1 cells. As a positive control, the parental 520C9 mAb chemically conjugated to rhIL-2 was shown to bind SKOV 3ip1 cells.
Figure 5 Determination of antigen-binding activity of H520C9sFv-hIL-2 fusion protein.
Antigen binding activity was measured by indirect ELISA using cultured SKOV 3ip1. Bound fusion protein was recognised with the anti-human IL2 polyclonal antibody EP100. The fusion proteins, H520C9sFv-hIL-2 and H520C9sFv-mhIL-2 were shown to bind to p185 positive cells SKOV 3ip1. A chemically-conjugated molecule containing the intact parental 520C9 mAb and rhIL-2(520C9-rhIL-2) was used as a positive control. As a negative control, mouse IgG failed to show any binding activity. The data are the mean ± SD from three separate experiments. bP < 0.01 vs mouse IgG; bP < 0.01 for 520C9-rhIL-2 vs H520C9sFv-hIL-2 and H520C9sFv-mhIL-2; dP < 0.01 vs mouse IgG; aP < 0.05 for 520C9-rhIL-2 vs H520C9sFv-hIL-2 and H520C9sFv-mhIL-2.
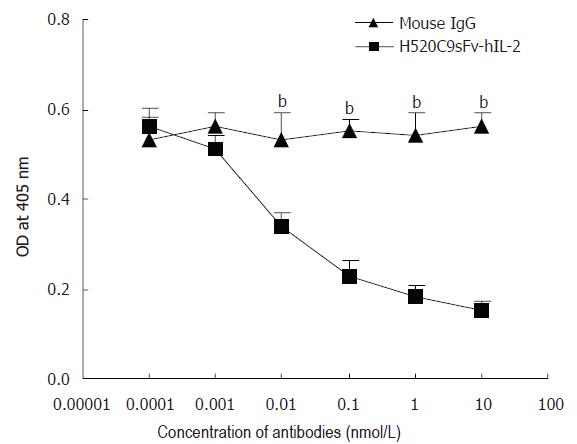
To confirm that the binding of H520C9sFv-hIL-2 fusion protein was specific for p185, SKOV 3ip1 cells were exposed to either the parental 520C9 mAb or normal mouse IgG prior to indirect ELISA. As shown in Figure 6, 520C9 mAb was able to inhibit the binding of the fusion protein to cells in a dose-dependent manner. In contrast, normal mouse IgG did not inhibit the binding of the fusion protein.
Figure 6 Anti-p185 mAb, 520C9, specifically blocks the binding of H520C9sFv hIL 2 fusion protein to SKOV 3ip1 cells.
SKOV 3ip1 cells, pre-exposed to serially diluted intact 520C9 monoclonal antibody or to mouse IgG (0.0001 to 10 nmol/L), then incubated with 10 nmol/L H520C9sFv hIL 2 fusion protein. Bound fusion protein was measured by indirect ELISA using the anti human IL 2 polyclonal antibody, EP100. 520C9 mAb caused a dose-dependent decrease in the binding of the fusion protein to SKOV 3ip1 cells. Control mouse IgG had no effect on the binding of the fusion protein. The data are the mean ± SD from three separate experiments. bP < 0.01 vs mouse IgG, P > 0.05 vs mouse IgG.
DISCUSSION
In this study, we report the construction, high efficient expression and characterisation of a recombinant humanized sFv antibody/hIL-2 fusion protein, H520C9sFv-hIL-2. The humanized sFv of 520C9 was constructed by introducing into a human sFv the amino acids from the complementarity-determining regions of mouse mAb 520C9, that recognize the HER/neu (c-erbB2) proto-oncogene product, p185[32]. Human IL-2 was then fused to the C-terminus of H520C9sFv by a flexible glycine linker. Following stable expression in 293 cells, this fusion protein was shown to retain both the immuno-stimulatory activities of IL-2 and the p185 binding characteristics of the parental 520C9 mAb. In the supernatant from stably transfected 293, the concentration of fusion protein cells was demonstrated to be at 102.0 ± 4.2 mg/L. This milligram scale expression makes it possible to prepare a large-scale fusion protein from mammalian cells, which is usually beneficial for keeping the bioactivities of fusion protein.
Our experiments demonstrated no loss of activity of IL-2 in the H520C9sFv-hIL-2 fusion protein as measured by IL-2-dependent cell proliferation and cytotoxicity assays, compared to rhIL-2 standard. This suggests that the fusion of IL-2 to the C-terminus of the humanized 520C9 sFv does not affect the biological activity of IL-2 and is consistent with previous studies describing the preparation of Fab’-IL-2 and SCA-IL-2 fusion proteins[33,34]. Retention of the IL-2 activity in H520C9sFv-hIL-2 may be partially due to the flexible glycine-rich linker between the C-terminus of sFv and the N-terminus of IL-2 which allows for proper folding of the IL-2 moiety, thereby enabling it to interact with its receptor. This is supported by previous findings with diphtheria toxin-IL-2 fusion proteins demonstrating the importance of the mobility of IL-2 in allowing its interaction to occur effectively[35].
We have developed a humanized sFv antibody/human IL-2 fusion protein, which was generated in mammalian cells with high efficacy and shown to retain both IL-2 activity and p185 binding affinity. The use of a humanized sFv as the fusion partner over other antibody-based molecules has the potential to reduce the immunogenicity of the fusion protein. In addition, the small size of the sFv fragment could likely improve its pharmacokinetic properties with respect to penetration of solid tumors and clearance. This bifunctional H520C9sFv-hIL-2 fusion protein may thus provide an effective method of targeting therapeutic doses of IL-2 to tumors or other targeted cells with significantly decreased side-effects of IL-2 and its immunogenicity.