Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3581
Revised: February 8, 2006
Accepted: February 18, 2006
Published online: June 14, 2006
AIM: To investigate the effect of exogenous ceramide-induced apoptosis on human colon carcinoma HT-29 cells.
METHODS: Light microscope, transmission electron microscope and fluorescence microscope were used to observe the morphology change of apoptosis in HT-29 cells. Agarose gel electrophoresis was performed to detect the DNA fragment. Mitochondrial function was detected by MTT assay. mRNA expression of Bcl-2 family gene members was determined by reverse transcription polymerase chain reaction (RT-PCR) assay.
RESULTS: After C2-ceramide treatment, typical characteristics of apoptosis, such as nuclear chromatin breakage, apoptotic body and DNA ladder, could be observed. After exposure to 50 μmol/L C2-ceramide for 12 and 24 h, cell apoptosis was 64.1% and 81.3% respectively, which had a time-and dose-effect relationship. Mitochondrial function started to decrease from 6 h after exposure to ceramide. Meanwhile, ceramide up-regulated or down-regulated the mRNA expression of Bcl-2 family gene members.
CONCLUSION: Ceramide induces apoptosis of human colon carcinoma HT-29 cells by affecting the expression of Bcl-2 family gene members and impacting the mitochondrial function.
- Citation: Zhang XF, Li BX, Dong CY, Ren R. Apoptosis of human colon carcinoma HT-29 cells induced by ceramide. World J Gastroenterol 2006; 12(22): 3581-3584
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3581.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3581
Ceramide (N-acetyl-sphingosine) is a fundamental unit of sphingomyelin which is a moiety of cytoplasmic membrane. Ceramide, also an important bioactive compound in vivo, can conduct specific signal transduction from cell surface receptor and environmental stress to nuclei, and then provoke multiple cytobiologic effects[1]. Meanwhile, ceramide is a common second messenger molecule participating in sphingomyelin cycle, apoptosis and differentiation of many cell types[2,3]. Apoptosis can be induced by surrounding stimulating factors, such as tumor necrosis factor α (TNF-α), endotoxin, FAS ligand, radiation, chemotherapeutics and heat shock, and is associated with ceramide. Recent studies indicate that ceramide has a close relationship with genesis and progression of digestive tract tumor. But little information about Bcl-2 family gene member and mitochondrial function is available concerning the effect of ceramide-induced apoptosis in colon carcinoma which is one of the most aggressive forms of cancer. Even ceramide could induce apoptosis of HT-29 cells. This study was to determine how ceramide induces apoptosis of human colon carcinoma cells and to discuss its mechanism.
C2-ceramide (N-acetyl-D-sphingosine) was purchased from Sigma, dissolved to 5 mmol/L in DMSO as a stock solution. Hoechest 33 258 and MTT were purchased from Sigma, dissolved in PBS solution. The two solutions were preserved at 4°C from light. Apoptosis DNA extract kit was from Shanghai Huashun Biotechnology Company. RPMI-1640 media, DMSO and fetal bovine serum (FBS) were bought from GIBCO. Trypsin and EDTA were obtained from Amresco.
HT-29 cells were provided by the Institute of Tumor Prevention and Cure in Beijing, China. Cells were cultured in RPMI-1640 media supplemented with 10% FBS and 2 mmol/L glutamine. Antibiotics added to the media were 100 U/mL penicillin and 100 U/mL streptomycin. Cells maintained in a humidified atmosphere of 95 mL/L air and 50 mL/L CO2 at 37°C were then passed at pre-confluent densities in a solution containing 0.25% trypsin and 0.02% EDTA. Exponentially growing cells were used throughout all experiments.
HT-29 cells were treated with C2-ceramide at final concentrations of 12.5, 25 and 50 μmol/L for 24 and 48 h respectively. DMSO served as negative control. During the procedure, cell morphology was observed under light microscope for different time.
Cells were collected and fixed with 4% glutaraldehyde in phosphate buffer overnight at 4°C. After post-fixation with 1% OsO4 in cacodylate buffer for 1h at 4°C, the pellet was dehydrated in graded ethanol solutions and embedded in Epon. Ultrathin sections of pellet were counterstained with uranyl acetate and lead citrate and observed under transmission electron microscope.
Cells (4 × 104) were treated as above, suspended with FBS and incubated with Hoechest 33 258 for 30 min at 37°C from light. Then the cells were observed under fluorescence microscope. Three hundred cells were counted and apoptotic cell rate was calculated.
Cells (2 × 106) were treated as above and collected. Fragmented DNA was extracted according to the manufacturer’s instructions of apoptosis DNA extract kit and underwent electrophoresis on 2 g/L agarose gel containing 0.5 g/L of ethidium bromide and visulized by UV transillumination.
Cells (1.5 × 104) were cultured in 96-well plates and treated with C2-ceramide for 1, 3, 6, 9, 12 and 24 h as above. Then MTT and DMSO were added by turns. Absorbance was determined with a multi-well plate reader at wavelength 570 nm.
Cells (1 × 106) were treated for 24 h as above. Total RNA was isolated from the treated cells with Trizol and reversely transcribed into cDNA with human specific primers for Bax, Bad, Bid, Bcl-2, Bcl-xl and β-actin. Sequences of primers are shown in Table 1. Briefly, 35 cycles of PCR amplification were performed at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s in a 25 μL reaction system. PCR products were confirmed by 1.5 g/L agarose gel electrophoresis and visualized by UV transillumination. mRNA expression of genes was assessed by correcting housekeeping gene β-actin, which served as an internal control.
| Gene | Primer sequence | Size (bp) | Temperature (°C) |
| Bax | Sense: 5’-CCAGCTGCCTTGGACTGT-3’ | 135 | 61 |
| Antisense: 5’-ACCCCCTCAAGACCACTCTT-3’ | |||
| Bcl-xl | Sense: 5’-GTAAACTGGGGTCGCATTGT-3’ | 146 | 60 |
| Antisense: 5’-TGGATCCAAGGCTCTAGGTG-3’ | |||
| Bcl-2 | Sense: 5’-GGCAATGTGACTTTTTCCAA-3’ | 137 | 55 |
| Antisense: 5’-GGCTGATATTCTGCAACACTG-3’ | |||
| Bad | Sense: 5’-AGGGCTGACCCAGATTCC-3’ | 178 | 60 |
| Antisense: 5’-GTGACGCAACGGTTAAACCT-3’ | |||
| Bid | Sense: 5’-GCTTCCAGTGTAGACGGAGC-3’ | 116 | 60 |
| Antisense: 5’-GTGCAGATTCATGTGTGGATG-3’ | |||
| β-actin | Sense: 5’-GTGGACATCCGCAAAGAC-3’ | 303 | 58 |
| Antisense: 5’-TCAACGCAATGTGGGAAG-3’ |
All statistical analyses were performed with SPSS 10.0 statistical package for Microsoft Windows. Data were expressed as mean ± SD for all measurements. P < 0.05 was considered statistically significant.
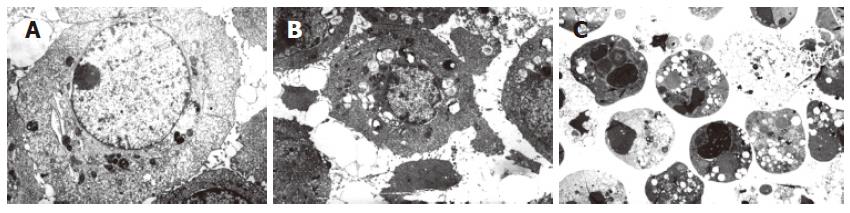
With increasing concentration of C2-ceramide and exposure time, HT-29 cells became round, atrophic and poorly adherent and floating cells increased under light microscope. Cells from control showed normal distribution and morphology in all cellular organelles except for a few necrotic cells under transmission electron microscope. The main ultra-microstructural changes seen in all treated groups were chromatin aggregation, mitochondrial denaturation and apoptotic body, as well as cytoplasmic compartments, swelling and disappearance of mitochondrial cristae, etc (Figure 1). The necrotic changes were more pronounced in cells treated with 50 μmol/L C2-ceramide.
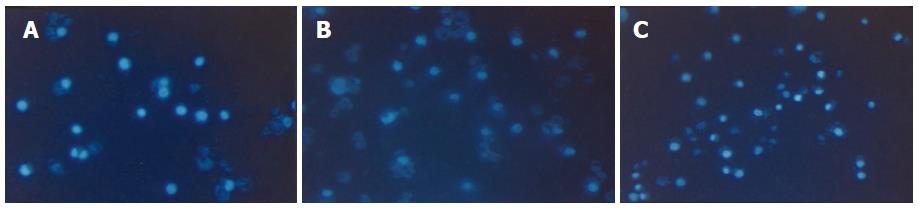
Apoptotic cells determined by Hoechest 33 258 assay increased in a time- and dose-dependent manner after treatment with C2-ceramide (Table 2). Normal cellular chromatin did not change and uniformly spread over the whole nuclei displaying diffusion uniformity fluorescence. However, apoptotic chromatin was identifiable by its scattered drop-like structure locating on the area of the original nuclei which displayed high brightness lump or punctiform fluorescence. The total size of apoptotic nuclei appeared smaller and more shrunken than the intact cells (Figure 2).
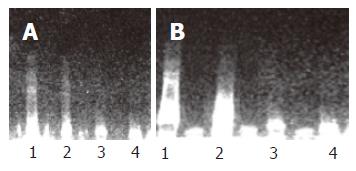
DNA ladder was seen through DNA agarose gel electrophoresis, especially pronounced in the cells treated with 50 and 25 μmol/L C2-ceramide for 12 and 24 h. Cells treated with 12.5 μmol/L C2-ceramide and DMSO kept their integrity, having no ladder appearance (Figure 3).
The absorbance value increased gradually with increased concentration of C2-ceramide and prolongation of exposure time. But the absorbance value after treatment with 50 and 25 μmol/L C2-ceramide was obviously lower than that in the control, showing statistical significance from 6 to 24 h, indicating that C2-ceramide could decrease mitochondrial function (Table 3).
| C2-ceramide (mmol/L) | 1 h | 3 h | 6 h | 9 h | 12 h | 24 h |
| 0 | 0.505 ± 0.061 | 0.596 ± 0.050 | 0.528 ± 0.083 | 1.088 ± 0.134 | 1.371 ± 0.066 | 1.770 ± 0.161 |
| 12.5 | 0.478 ± 0.044 | 0.609 ± 0.048 | 0.502 ± 0.029 | 0.833 ± 0.149a | 1.036 ± 0.122 | 1.560 ± 0.220 |
| 25 | 0.487 ± 0.051 | 0.568 ± 0.048 | 0.404 ± 0.033a | 0.646 ± 0.158a | 0.760 ± 0.086a | 1.484 ± 0.346a |
| 50 | 0.490 ± 0.054 | 0.541 ± 0.049 | 0.345 ± 0.050a | 0.456 ± 0.071a | 0.708 ± 0.073a | 1.342 ± 0.061a |
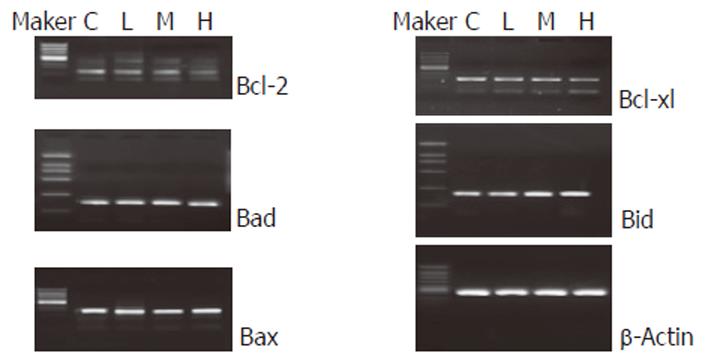
After cells were treated with C2-ceramide for 24 h, the mRNA expressions of Bax, Bad and Bid genes were up-regulated. However, the expressions of Bcl-2 and Bcl-xl were down-regulated (Figure 4). Moreover, the ratio of Bcl-2 to Bax in 50 μmol/L C2-ceramid group was less than 1.
Recently, the importance of ceramide in cell metabolism has been broadly investigated. The biological effect of ceramide on different cell lines is different. The role of ceramide-induced apoptosis has been confirmed[4]. Recent studies indicate that ceramide is a common second messenger molecule of apoptosis. Apoptosis induced by stimulating factors is mediated by ceramide from sphingomyelin circulation way[5,6]. The effect of ceramide on apoptosis has been studied extensively in neoplastic cells but rarely in solid tumor cells. Ceramide is closely related with genesis and progression of digestive tract tumor. Decreased ceramide content increases the risk of developing digestive tract tumor since ceramide content in human colon carcinoma cells is lower than that in normal colon mucosa cells[7].
To some extent, tumor chemotherapy is to induce apoptosis. In this experiment, human colon cancer cells were exposed to exogenous C2-ceramide. Results indicated that C2-ceramide could induce typical characteristics of apoptosis, such as nuclear chromatin break and apoptotic body as well as DNA ladder in a time-and dose-dependent manner. Succinate dehydrogenase (SDH) in the mitochondria is an index of cellular respiration and energy, which reflects mitochondrial function. MTT assay suggested that C2-ceramide could decrease mitochondrial function. By studying Bcl-2 family gene members, we also found that C2-ceramide could up-regulate or down-regulate the mRNA expression of these genes, suggesting that exogenous C2-ceramide induces apoptosis of human colon carcinoma cells in vitro by affecting the expression of Bcl-2 family gene members and damaging the mitochondrial functions.
C2-, C6 and C8-ceramides could induce apoptosis of cell lines, but dihydroxy-ceramide lacking C4-C5 trans-double bond located in basal framework of sphingolipid could not induce apoptosis[8].
Studies also indicate that ceramide has many target sites inducing apoptosis, such as ceramide-activated protein kinase (CAPK)[9], ceramide-activated protein phosphatase (CAPP)[10], protein kinase C (PKC) family[11], stress-activated protein kinase/c-JUN N-terminal kinase (SAPK/JNK)[12] and caspase cascade reaction[13]. There is a body of evidence that mitochondria are involved in apoptosis, for example, release of cytochrome C from mitochondria triggers apoptosis[14-16].
In conclusion, mitochondria is the target of ceramide. The importance and mechanism of mitochondria in ceramide-induced apoptosis need to be further studied.
| 1. | Perry DK, Hannun YA. The role of ceramide in cell signaling. Biochim Biophys Acta. 1998;1436:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 242] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125-3128. [PubMed] |
| 3. | Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 643] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1336] [Cited by in RCA: 1415] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 5. | Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Selzner M, Bielawska A, Morse MA, Rüdiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233-1240. [PubMed] |
| 8. | Ahn EH, Schroeder JJ. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp Biol Med (Maywood). 2002;227:345-353. [PubMed] |
| 9. | Stoica BA, Movsesyan VA, Knoblach SM, Faden AI. Ceramide induces neuronal apoptosis through mitogen-activated protein kinases and causes release of multiple mitochondrial proteins. Mol Cell Neurosci. 2005;29:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Fishbein JD, Dobrowsky RT, Bielawska A, Garrett S, Hannun YA. Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J Biol Chem. 1993;268:9255-9261. [PubMed] |
| 11. | Signorelli P, Luberto C, Hannun YA. Ceramide inhibition of NF-kappaB activation involves reverse translocation of classical protein kinase C (PKC) isoenzymes: requirement for kinase activity and carboxyl-terminal phosphorylation of PKC for the ceramide response. FASEB J. 2001;15:2401-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP. Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res. 2004;64:7852-7856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Zhao S, Yang YN, Song JG. Ceramide induces caspase-dependent and -independent apoptosis in A-431 cells. J Cell Physiol. 2004;199:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 282] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1092] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 16. | Li JM, Zhou H, Cai Q, Xiao GX. Role of mitochondrial dysfunction in hydrogen peroxide-induced apoptosis of intestinal epithelial cells. World J Gastroenterol. 2003;9:562-567. [PubMed] |
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH