Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3461
Revised: February 8, 2005
Accepted: February 18, 2006
Published online: June 14, 2006
Patients who are chronically infected with the hepatitis C virus often develop chronic liver disease and assessment of the severity of liver injury is required prior to considering viral eradication therapy. This article examines the various assessment methods currently available from gold standard liver biopsy to serological markers and imaging. Ultrasound is one of the most widely used imaging modalities in clinical practice and is already a first-line diagnostic tool for liver disease. Microbubble ultrasound contrast agents allow higher resolution images to be obtained and functional assessments of microvascular change to be carried out. The role of these agents in quantifying the state of hepatic injury is discussed as a viable method of determining the stage and grade of liver disease in patients with hepatitis C. Although currently confined to specialist centres, the availability of microbubble contrast-enhanced ultrasound will inevitably increase in the clinical setting.
- Citation: Grier S, Lim AK, Patel N, Cobbold JF, Thomas HC, Cox IJ, Taylor-Robinson SD. Role of microbubble ultrasound contrast agents in the non-invasive assessment of chronic hepatitis C-related liver disease. World J Gastroenterol 2006; 12(22): 3461-3465
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3461
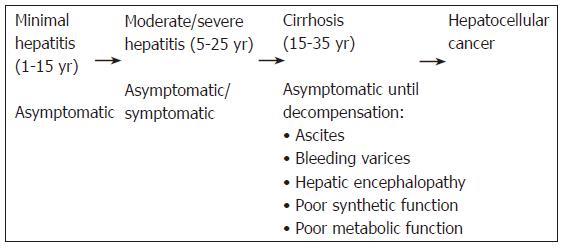
It is estimated that 170 to 200 million people (around 3% of the global population) are chronically infected with the hepatitis C virus (HCV), while around 4 million people are newly infected each year[1]. There is a great degree of variation in prevalence, ranging from less than 1% in the United Kingdom to more than 20% in Egypt. In the developed world, the relatively low prevalence is mostly the result of intravenous drug abuse, while a World Health Organisation (WHO) anti-schistosomal vaccination programme in Egypt in the 1970s and other vaccination programmes in Mongolia and Bolivia have led to much higher rates in those countries[2]. Between 55% and 85% of those infected go on to develop chronic liver disease and even then, many remain asymptomatic, while the rest suffer primarily from chronic fatigue[3]. Around 20% of patients with fibrosis develop cirrhosis and 5% of these are susceptible to developing hepatocellular cancer each year (Figure 1)[4]. A number of factors have been linked to the progression of chronic hepatitis, with some being implicated not only in advancing the progression, but also worsening the overall prognosis[5]. These factors include age at infection greater than 55 years, male sex, excessive alcohol consumption, a long history of cigarette smoking and the presence of hepatic steatosis, obesity and diabetes mellitus (the metabolic syndrome)[5].
An assessment of the severity of hepatic involvement is obviously required prior to considering antiviral treatment regimens with interferon and ribavirin in patients with hepatitis C. In addition, owing to the variable disease progression, the potentially life-threatening complications of end-stage liver disease and the development of hepatocellular carcinoma, it is also imperative to monitor hepatitis C-infected patients with established cirrhosis.
For many years, the gold standard for assessing chronic hepatitis has been histological examination of liver biopsy material. However, this invasive procedure is not without risks with a small, but defined mortality rate and it has a significant error rate, sampling less than 1/50 000th of the liver volume even in cases of diffuse disease[6-8]. As a result of the problems associated with biopsy, there has been a steady drive in recent times to find an effective non-invasive method of evaluating liver injury, which has led to major developments in both serological markers of disease and imaging.
An ideal serological marker for assessing liver injury should be liver-specific, easy to use and allow measurement of the stage of disease using the degree of fibrosis, the activity of matrix deposition and the activity of matrix removal[8]. These can be broadly classified into two groups. The first (Group 1 in Table 1) are those which are indirect markers of fibrosis, reflecting alterations in hepatic function, and include PGAA {prothrombin index, gamma glutamyl transpeptidase (γGT), apolipoprotein A1 and α2-macroglobulin}[8], AST/ALT (aspartate aminotransferase and alanine aminotransferase)[9], FibroTest (α2-macroglobulin, α2-globulin, γ-globulin, apolipoprotein A1, γGT and total bilirubin)[10] and APRI (AST to platelet ratio index)[11]. The second (Group 2 in Table 1) are those which are direct markers of fibrosis, reflecting extracellular matrix metabolism (Group 2 in Table 1)[8]. The current issue is that many of these proposed panels have not been widely validated around the world and often remain championed largely by the research groups that initially proposed them. Currently, none fulfils all the criteria for an ideal serological marker and none has yet achieved the levels of sensitivity and specificity seen with liver biopsy.
| Group | Marker/panel | Comments |
| 1 | Prothrombin index | Highest diagnostic accuracy (cirrhosis) 86% |
| 1 | PGAA | Diagnostic accuracy 80% |
| 1 | AST/ALT ratio | Increase strongly correlated with decrease in hepatic function AST/ALT > 1 can diagnose cirrhosis with sensitivity of 77.8% and specificity 96.9%, but misclassified 7% as having cirrhosis and failed to diagnose 11% |
| 1 | FibroTest | Sensitivity 75%, specificity 85% |
| 1 | APRI | Predicts fibrosis in 51% patients Predicts cirrhosis in 81% patients |
| 2 | Hyaluronic acid (HA) | Only group 2 marker to show encouraging results, with a positive predictive value similar to Child-Pugh |
Of all the proposed non-invasive alternatives to liver biopsy, imaging has shown some promise. Using routine greyscale ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI), it has been shown that a small right hepatic lobe, with relatively enlarged left and caudate lobes, are reliable markers of cirrhosis, having a sensitivity of 84%, specificity of 100% and diagnostic accuracy of 94%. These figures are well beyond any achieved by the many serological markers[8].
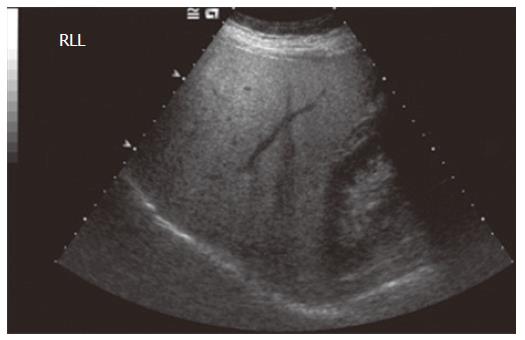
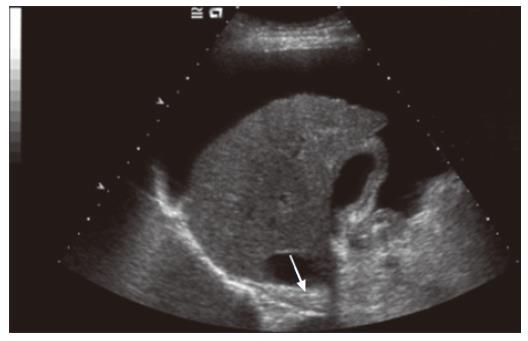
Ultrasound is one of the most widely used imaging modalities in clinical practice. The major attraction is that it is already the first line of imaging in the diagnosis of liver disease and is of particular interest, as it has been shown to be complementary in patients with equivocal biopsy results or where biopsy is contraindicated, but cirrhosis is suspected[8]. It is also used to screen patients with chronic hepatitis for hepatocellular cancer, enabling detection and characterisation of tumors[12]. In patients with cirrhosis, it is possible to demonstrate nodularity of the liver surface and parenchyma and sometimes atrophy of the right lobe[13], but for the most part, standard grey-scale imaging does not render much information on the extent of fibrosis in pre-cirrhotic disease. The most accurate diagnoses which can be made on grey-scale imaging are those of hepatic steatosis, demonstrated by parenchymal hyperechogenicity (Figure 2), and the presence of a nodular surface in cirrhosis (Figure 3). The need to discriminate pre-cirrhotic liver disease non-invasively has driven the development of additional ultrasound techniques, including elastography and a group of microbubble ultrasound contrast agents, in order to achieve better resolution images and allow a degree of hepatic functional assessment.
Transient elastography has recently been used to characterize the stiffness of liver tissue in vivo, which has been correlated with the histological grade of fibrosis in hepatitis C[14]. A probe vibrating at a predetermined amplitude and frequency is placed against skin overlying the liver. The resultant shear wave is propagated through tissue at a velocity dependent on its stiffness. The velocity can be measured by one-dimensional ultrasound along the axis of the transmitted wave. Although correlation is good overall, there is significant overlap of stiffness as measured by elastography between the different stages of fibrosis. Elastography has been used in conjunction with the serological markers, FibroTest (Table 1 for details) and the aspartate aminotransferase (AST) to platelet ratio (APRI) to evaluate liver fibrosis. There was correlation with histology, but significant overlap of elastography values between stages of fibrosis[15,16]. Further larger scale studies are required using this methodology.
In the late 1960s, a group of echocardiographers discovered, quite by accident, that the microscopic bubbles of air which enter the circulation during intravenous injections produce strong echoes, where normally none is seen[17,18]. This discovery led to the development of the first type of microbubble contrast agents for ultrasound, which became available in the mid-1990s. Microbubbles are tiny, gas-filled bubbles with a shell (made from denatured albumin, phospholipids, surfactant or cyanoacrylate) which measure less than 10 μm in diameter and are small enough to pass through the cardiopulmonary circulation and also cross capillary beds[19].
In simple terms, microbubbles are echo-enhancers, which enhance both grey-scale and Doppler ultrasound signals by up to 25 dB, giving an increase of more than 300 folds[20]. The first microbubble agent to be licensed for use in the United Kingdom was Levovist® in 1997. Although it exhibited a degree of liver specificity, it had a short half-life and the bubbles were mostly destroyed by liver, meaning that only one pass through the liver was possible. The introduction of SonoVue®, a third-generation microbubble agent, in 2001 allowed real-time contrast-enhanced imaging and multiple passes through the liver.
As with all pharmaceutical agents, microbubble agents have to pass rigorous safety tests in order for them to be licensed. Extensive trials have established an excellent overall safety record with few significant adverse effects[20]. However, there are some concerns that disruption of their outer shells produces local bioeffects such as sonoporation (subcellular membrane damage) and cell lysis at diagnostic frequencies and that these effects are enhanced by perfluorocarbon gases. Despite this, no effects of these processes have been observed in humans[20]. Concern about administering volumes of gas into the blood stream has been shown not to be an issue as the amount given is under 200 μL, too small to exhibit an effect[21]. Overall, it has been shown that the safety of microbubbles compares favourably to that of conventional radiographic contrast agents and those used in contrast-enhanced MRI[21].
Microbubbles have a multitude of clinical uses, but are best known for imaging the vasculature, contrast-enhanced echocardiography, imaging the liver and functional studies. In these functional studies, following an intravenous bolus injection, their passage can be tracked through a tissue or organ and used to generate “transit time” curves in much the same way as those in nuclear medicine, CT and MRI are produced[20].
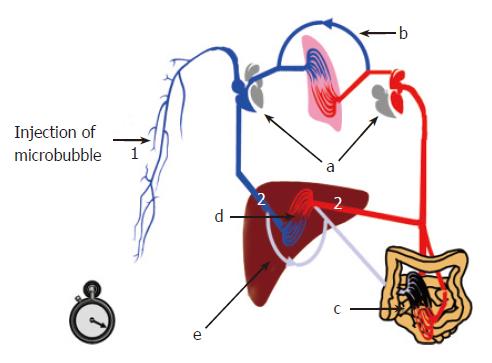
One of the major applications of transit times is in the assessment of liver disease and the non-invasive diagnosis of cirrhosis[21]. Severe liver disease leads to characteristic haemodynamic changes affecting hepatic blood flow. The development of a hyperdynamic circulation (increased cardiac output, reduced systemic vascular resistance, altered circulating vasoactive factors-glucagons and prostaglandins), pulmonary arteriovenous shunts, venous collaterals (due to increased portal pressure), intrahepatic shunts (between hepatic artery, portal vein and hepatic veins), and arterialisation of the liver capillary beds, results in the early arrival of a bolus of microbubbles injected peripherally (Figure 4). The hepatic vein transit time (HVTT) is the difference between the hepatic artery and the hepatic vein arrival times.
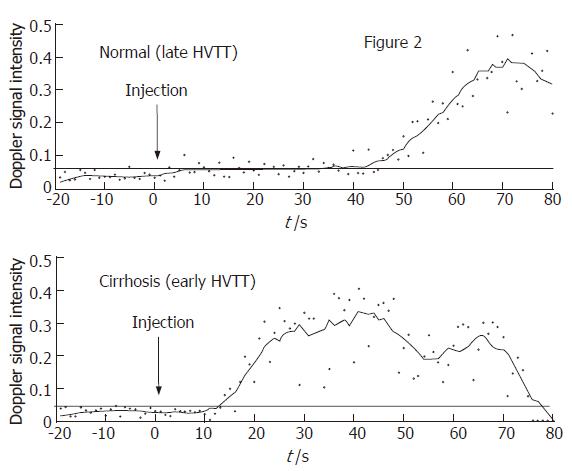
Many of the published studies[22-24] assessing HVTT in patients with chronic liver disease have employed measurements using the spectral Doppler technique, where the arrival time is calculated as a rise in Doppler intensity 10% above baseline. This requires specialized software to evaluate the HVTT, but is relatively straight-forward, as shown in Figure 5. The figure demonstrates a normal hepatic vein transit time (43 s) in a patient without significant liver disease (upper graph, Figure 5) and a comparative early HVTT in a patient with cirrhosis (14 s) (lower graph, Figure 5). There is a much shorter HVTT in the patient with cirrhosis. It is important to note that 20 s of baseline is collected before the injection of the microbubble contrast agent. The continuous horizontal line denotes a Doppler intensity 10% above baseline and hence the HVTT is taken as the intersection of this line with that of the Doppler intensity trace (Figure 5).
The development of newer more stable microbubbles, such as SonoVue® (Bracco, Milan, Italy) and improvement in ultrasound capabilities have allowed ‘real-time’ evaluation of the transit times which can be readily visualized arriving in the hepatic arteries and veins (Figures 5 and 6) without the need for ‘off-line’ processing.
A typical, simplified transit time assessment would now involve the injection of a bolus of the microbubble agent into an antecubital fossa vein and measurement of the time taken for the contrast agent to be seen in the liver. The first flash of microbubbles seen in the hepatic arteries is recorded as the hepatic artery arrival time. A continuous stream of microbubbles in the hepatic vein is recorded as the hepatic vein arrival time. The hepatic vascular transit time (HVTT) is thus the difference between the hepatic artery and the hepatic vein arrival times.
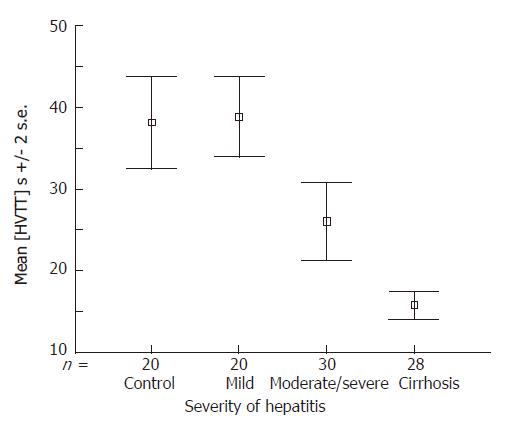
It has been shown that these transit times can be linked to progression in the Child-Pugh score, becoming shorter as the liver disease worsens[22]. Pre-cirrhotic disease due to hepatitis C can now be classified into mild (≥ 28 s) or moderate to severe (≥ 22 s, < 28 s) according to transit times[20]. A recent study has shown that a transit time of ≥ 21 s is 100% sensitive and 96% specific for cirrhosis, making it a viable alternative when liver biopsy is contraindicated. It is also perhaps a method of monitoring disease progression in response to treatment[23]. Figure 6 is adapted from a recent study by Lim et al[23] which shows a progressive shortening of the HVTT with increasing severity of liver disease in a cohort of patients with HCV-related liver disease. There is clear separation between the groups (categorisation of patients was carried out according to the Ishak histological scoring system[25]). Importantly, there is a clear separation, not only between the patients with moderate hepatitis from those with cirrhosis, but also in the pre-cirrhotic groups, where there is good separation of patients with mild hepatitis from those with moderate hepatitis[23]. This may be clinically relevant and demonstrates for the first time the possibility that ultrasound techniques can actually monitor the severity of pre-cirrhotic disease non-invasively.
Levovist is at present the most commonly used microbubble agent, but there are several newer agents, such as SonoVue® (Bracco, Milan, Italy) or Definity® (Bristol-Myers-Squibb, New York, USA), which have different chemical properties to Levovist®, but also provide enhanced Doppler intensity when they arrive in the hepatic veins. It remains to be shown whether the HVTT for these agents are similar and whether they can be used interchangeably. A benefit of using these newer agents is that their arrival within a hepatic vein can be observed in ‘real-time’, using low acoustic power two-dimensional harmonic scanning modes. The main benefit of using these modes is that it would not require computer-aided post-processing of the data to calculate the HVTT, thus making it accessible to any department with a modern ultrasound machine.
There are clearly a number of very different methods currently available for the monitoring of hepatitis C-related liver disease. Although liver biopsy remains the gold standard, it is a highly invasive procedure that is not without risk and is open to sampling error, particularly in disease where there is patchy involvement. There is a continual drive towards minimally or non-invasive testing, and therefore, alternatives to liver biopsy are being actively sought.
The use of serological markers remains somewhat confused, with prothrombin index having the highest diagnostic accuracy, despite many other panels being suggested. Further research by independent teams is required to understand exactly what role these markers can perform.
Developments in imaging technology in the past decade have yielded sensitivities and specificities similar to that of liver biopsy for the first time. Ultrasound continues to be the primary imaging modality of choice when investigating liver disease. Although currently limited to a few specialist centers, microbubble contrast agents will inevitably become much more widely used. Hepatic vein transit time measurement using an ultrasound microbubble agent is a simple, non-invasive test which can be used to stage and grade pre-cirrhotic liver disease with clear separation between mild hepatitis and cirrhosis, and statistically significant differences between these two groups and moderate to severe hepatitis. The response to treatment using hepatic vein transit times needs to be assessed in future longitudinal studies, but the methodology is potentially translatable as an efficient, quick and discriminative test for the assessment of disease severity in hepatitis C to any ultrasound department anywhere in the world.
| 1. | World Health Organisation. Hepatitis C factsheet no. 164, revised October. 2000; Available from: http//www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 686] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | National Institute of Clinical Excellence (N. I.C.E.). Guidance on the use of ribavirin and interferon alpha for hepatitis C. October. 2000; Available from: http//www.nice.org.uk/pdf/RIBAVIRIN-guidance14.pdf. |
| 5. | Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Poynard T, Imbert-Bismut F, Munteanu M, Messous D, Thabut D, Ratziu V, Benhamou Y. Biomarkers as non-invasive assessment of hepatic fibrosis in chronic hepatitis C. J Gastroenterol Hepatol. 2004;19:S236-S245. [DOI] [Full Text] |
| 7. | Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36:S57-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 423] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1038] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 11. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3346] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 12. | Kono Y, Mattrey RF. Ultrasound of the liver. Radiol Clin North Am. 2005;43:815-826, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023-1032; quiz 1033-1034;. [PubMed] |
| 14. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1097] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 15. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1861] [Article Influence: 88.6] [Reference Citation Analysis (2)] |
| 16. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Blomley M, Cosgrove D. Microbubble echo-enhancers: a new direction for ultrasound. Lancet. 1997;349:1855-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Becher H, Burns PN. Handbook of contrast echocardiography. LV function and myocardial perfusion (Electronic Version). Berlin: Springer 2000; 2-42 Available from: http//www.sunnybrook.utoronto.ca/EchoHandbook/. |
| 19. | Harvey CJ, Pilcher JM, Eckersley RJ, Blomley MJ, Cosgrove DO. Advances in ultrasound. Clin Radiol. 2002;57:157-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Harvey CJ, Blomley MJ, Eckersley RJ, Cosgrove DO. Developments in ultrasound contrast media. Eur Radiol. 2001;11:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001;322:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 255] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Blomley MJ, Lim AK, Harvey CJ, Patel N, Eckersley RJ, Basilico R, Heckemann R, Urbank A, Cosgrove DO, Taylor-Robinson SD. Liver microbubble transit time compared with histology and Child-Pugh score in diffuse liver disease: a cross sectional study. Gut. 2003;52:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Lim AK, Taylor-Robinson SD, Patel N, Eckersley RJ, Goldin RD, Hamilton G, Foster GR, Thomas HC, Cosgrove DO, Blomley MJ. Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut. 2005;54:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3839] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
S- Editor Wang J L- Editor Kumar M E- Editor Bi L