Published online Jun 7, 2006. doi: 10.3748/wjg.v12.i21.3386
Revised: October 28, 2005
Accepted: November 10, 2005
Published online: June 7, 2006
AIM: To explore rectal nitric oxide (NO) as biomarker of treatment response in ulcerative colitis (UC) and Crohn’s disease (CD), and examine relationships between rectal NO, mucosal expression of NO synthases (NOS), and pro-inflammatory cytokines.
METHODS: Twenty-two patients with UC and 24 with CD were monitored during steroid treatment. Rectal NO levels were measured and clinical activities were assessed on days 1, 3, 7 and 28. Mucosal presence of NOS and pro-inflammatory cytokines were analyzed by immunohistochemistry and RT-PCR.
RESULTS: Active UC and CD displayed markedly increased rectal NO levels (10 950 ± 7610 and 5 040 ± 1 280 parts per billion (ppb), respectively) as compared with the controls (154 ± 71 ppb, P < 0.001). Rectal NO correlated weakly with disease activity in both UC and CD (r = 0.34 for UC and r = 0.48 for CD, P < 0.01). In 12 patients, a steroid-refractory course led to colectomy. These patients had only slightly increased NO levels (UC: 620 ± 270 ppb; CD: 1260 ± 550 ppb) compared to those with a therapeutic response (UC: 18 860 ± 530 ppb, P < 0.001; CD: 10 060 ± 3200 ppb, P < 0.05).
CONCLUSION: Rectal NO level is a useful biomarker of treatment response in IBD as low NO levels predicts a poor clinical response to steroid treatment.
-
Citation: Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M, Lundberg JO, Hellström PM. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: Responders
versus nonresponders. World J Gastroenterol 2006; 12(21): 3386-3392 - URL: https://www.wjgnet.com/1007-9327/full/v12/i21/3386.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i21.3386
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory diseases sharing a clinical course with flares of disease, characterized by an increase of symptoms due to increased inflammatory activity of the intestinal mucosa. Symptom-based clinical activity indices are today’s standard methods applied to monitor disease activity in clinical trials, but rarely used in clinical practice. Available indices have been criticized for depending almost exclusively on clinical features that are often subjective. The use of systemic markers of inflammation, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), platelet count and white blood cell (WBC) count are commonly used in clinical practice, but correlation to ongoing intestinal inflammation is poor[1,2]. An approach of direct assessment of mucosal inflammation has been promising, such as measurements of tumor necrosis factor (TNF)-α and interleukin (IL)-1β[3] and intestinal permeability tests[4]. The implementation of these tests is, however, restricted by a complicated analysis. The development of a more feasible objective marker of mucosal inflammation is, therefore, warranted. Data on local measurements of inflammatory products such as calprotectin, lactoferrin and nitric oxide (NO) in UC and CD have been promising[5].
We use a minimally invasive method employing chemiluminescence to measure rectal NO levels in conditions with inflamed intestinal mucosa[6,7]. Increased NO generation has been demonstrated in both UC and CD[6,8,9]. The excessive formation of NO is elaborated by inducible nitric oxide synthase (iNOS)[10,11]. Some groups have also reported increased endothelial NOS (eNOS) activity in inflamed mucosa in patients with UC[12] and iNOS expression restricted to epithelial cells[13,14]. Other groups have demonstrated iNOS expression also in macrophages and granulocytes of the lamina propria[10,11].
The activation of iNOS is dependent on the transcription factor nuclear factor (NF)-κB, which is activated by bacterial products (e.g., endotoxins) and pro-inflammatory cytokines, such as TNF-α, IL-1β and interferon (INF)-γ. These pro-inflammatory cytokines act in synergy to stimulate NO production[15]. The aim of the present study was to investigate rectal NO levels as a biomarker of response in UC and CD during glucocorticosteroid (GCS) treatment, using established clinical indices as gold standard for clinical activity. We also assessed mucosal immunoreactivity to the cytokines TNF-α, IL-1β and INF-γ as well as activation of eNOS, iNOS and neuronal NOS (nNOS).
Patients treated with prednisolone (0.5-1 mg/kg orally) at the Karolinska University Hospital for active UC or CD were eligible to enter this study. Forty-six consecutive patients, 22 with UC and 24 with CD, diagnosed by conventional endoscopic, radiological and histological criteria of IBD[16] were recruited to this study. Diagnosis of UC or CD was confirmed for all included patients at follow-up one-year after completion of the study. The majority of patients had concomitant medication with aminosalicylates, whereas no patient was on immunomodulators at baseline (Table 1).
| Ulcerative colitis | Crohn's disease | |
| (n = 22) | (n = 24) | |
| Sex (M/F) | 13/9 | 14/10 |
| Age (yr) (mean and range) | 41 (18 – 78) | 42 (20 – 69) |
| Duration (yr) (mean and range) | 7.4 (0 – 30) | 9 (0 – 28) |
| Smoking habits (n) | ||
| Active | 2 | 3 |
| Former | 6 | 8 |
| Non-smoker | 7 | 9 |
| Unknown | 7 | 4 |
| Concomitant medication (n) | ||
| Aminosalicylates | 18 | 12 |
| Local steroids | 6 | 2 |
| Site of disease | ||
| Ileocolonic | 24 | |
| Rectum | 3 | |
| Proctosigmoiditis | 6 | |
| Extensive/total | 13 | |
| Disease activity index | ||
| (mean and range) | 9.4 (5 – 12) | |
| Harvey-Bradshaw index | ||
| (mean and range) | 14.9 (4 – 29) | |
| Endoscopic score | ||
| (mean and range) | 2.2 (1-3) | 2.1 (0-3) |
The patients were studied at four different occasions during the first month of treatment, before onset of prednisolone treatment (d 1), and at follow-up during ongoing treatment on days 3, 7 and 28. A flexible sigmoidoscopy was performed at baseline, and at all follow-up visits. Disease activity was assessed using the Disease Activity Index (DAI)[17] for the patients with UC. The Harvey-Bradshaw Index (HBI)[18] was used for the patients with CD. The endoscopic classification was done according to the DAI score also for the CD patients.
At the last visit (d 28), the patients were divided into responders (remission) and non-responders (no remission) for further subgroup analysis. Remission was defined as DAI ≤ 2 in UC, and HBI ≤ 4 in CD, whereas non-responders were defined as DAI ≥ 3 in UC and HBI ≥ 5 in CD, respectively.
Two different control groups were used: the control for immunohistochemistry analyses consisted of six individuals undergone control colonoscopy after prior polypectomy, and the control for rectal NO consisted of 25 healthy volunteers with no history of gastrointestinal symptoms or abdominal surgery.
The study was approved by the Karolinska Institutet Ethics Committee. Written informed consent was taken from all patients.
NO was measured with a chemiluminescence analyzer (CLD 700, Eco Physics, Dürnten, Switzerland). The detection limit for NO was 1 part per billion (ppb). The analyzer was calibrated at known concentrations (100-10 000 ppb) of NO in nitrogen gas (AGA, Lidingö, Sweden), administered through an electromagnetic flow controller (Environics, Middletown, CT, USA). The chemiluminescence assay is highly specific for NO without interference from other nitrogen oxides[19]. For sampling of rectal gas, we applied an all-silicon catheter (Argyle®, Sherwood Medical, Tullamore, Ireland) inserted into the rectum, using lubrication gel, free of local anesthetics, to a level 10 cm above the anal sphincter[7]. The balloon of the catheter was then inflated with 10 mL of ambient air containing less than 5 ppb of NO, and left for 10 min to equilibrate with gases in the rectum. Thereafter, the gas was withdrawn from the catheter balloon and diluted to a final volume of 50 mL before chemiluminescence analysis with correction for dilution. Analyses were performed within 15 min of sampling[7]. In cases where measurements exceeded the upper detection limit, further dilution steps were made in order to measure NO within the calibrated range.
Mucosal biopsies were sampled at sigmoidoscopy. Biopsy specimens were always taken in the vicinity of lesions or ulcerations. In case of colectomy (n =12), the surgical specimens were additionally used for analyses. Biopsies were kept in Histocon (Histolab, Gothenburg, Sweden) on ice and snap-frozen in liquid nitrogen within 30 min. Approximately 6 μm thick cryostat sections were mounted on gelatin-coated glass slides, and stored at -80 °C. Before staining, the slides were thawed at room temperature and subsequently fixed in cold 20 g/L formaldehyde in phosphate-buffered saline (PBS). All following incubation and washing steps were performed in PBS supplemented with 1 g/L saponin (Sigma Chemicals, St Louis, MO, USA) to permeabilize cellular membranes[20]. Peroxidase activity was blocked with 3 g/L hydrogen peroxidase and 1 g/L sodium azide in PBS-saponin[21]. Human and goat serum (Vector Laboratories Inc, Burlingame, CA, USA) and avidin/biotin blocking kit (Vector Laboratories) were used to block unspecific bindings. The sections were incubated overnight at 4°C with mouse monoclonal antibodies to iNOS (NOS-IN 20 mg/L, Sigma), nNOS (NOS-BI 73 mg/L, Sigma), eNOS (NOS-EI 17 mg/L, Sigma), IL-1β (2D8 1.4 mg/L, ImmunoKontact, Abingdon, Oxon, UK), TNF-α (Mab I 10 mg/L + Mab II 14 mg/L, Pharmigen, San Diego, CA, USA ), and to INF-γ (7-B6- 10 mg/L + 1-DIK 10 mg/L, Mabtech AB, Nacka, Sweden). Mouse IgG1 (28 mg/L, Dako, Glostrup, Denmark) served as an isotype-matched negative control. Biotinylated goat anti- mouse IgG1 (4 mg/L, Caltag laboratories, Burlingame, CA, USA) was used as secondary antibody, except for the eNOS staining (an IgA antibody) where it was substituted with biotinylated goat anti-mouse immunoglobulin (11 mg/L, Dako). The biotinylated secondary antibodies were followed by horse-radish peroxidase-conjugated avidin/biotin-complex (Vectastain, ABC-elite, Vector Laboratories). Positive peroxidase staining was developed with 3, 3´-diaminobenzidine (DAB peroxidase substrate kit, Vector Laboratories) and counterstained with haematoxylin (Histolab).
All microscopic evaluations were performed by one investigator (T.L.) who was blinded to the clinical data as well as analyzed parameters (eNOS, nNOS, iNOS, TNF-α, IL-1β, INF-γ and IgG1). To validate the observer’s quantification, eight randomly chosen sections were additionally analyzed by a second observer (C. J.). Absolute correlation was seen between the two observers. Sections were analyzed using light microscope (Nikon Ltd, Tokyo, Japan). For each section, three different grid areas rich in positive cells of satisfactory technical quality were chosen for quantitative analysis of NOS and cytokine immunohistochemistry. Due to some background staining of the epithelial cells, we restricted our quantitative analysis to the lamina propria. For each area, the number of positive cells was counted at high-power magnification (× 400), thereafter the total number of positive cells was divided with the total grid area. For each section, the result was expressed as the mean number of positive cells per one grid area. For comparison, the percentage of positive cells in the lamina propria was also calculated. A high correlation between immunohistochemistry quantification expressed as positive cells per grid area and percentage positive cells of total cell number in lamina propria was observed (r = 0.98, P < 0.001).
Colonic biopsies were taken from four patients with CD and two with UC. The biopsies were collected from both normal and inflamed parts of the colon in each patient. Tissue samples were immediately placed in RNAlater (Qiagen, Hilden, Germany), stored for 24 h at 2-8°C and then at -80°C.
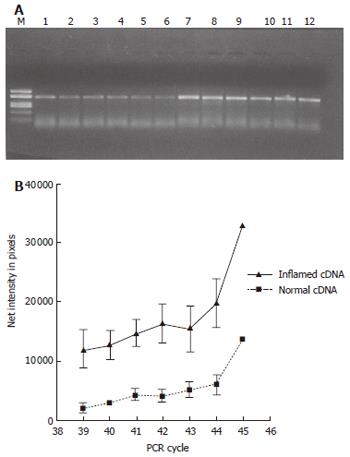
After thawing, total RNA was isolated from the biopsies using the RNeasy mini kit (Qiagen). cDNA was synthesized using oligo (dT)20 primers and SuperScript III enzyme (Invitrogen, Carlsbad, CA, USA). Semi-quantitative RT-PCR was performed using cDNA normalized against the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PCR products obtained with primers specific for the human iNOS gene (sense 5´-CCAAAAGGGTCATCATCTCT-3´; antisense 5´- CCTGCTTCACCACCTTCTTG-3´) (GenBank accession number AF049656) in the linear amplification range (39 to 45 cycles) were electrophoresed on a 15 g/L agarose gel, photographed and bands quantified (Kodak Gel Logic 100 Imaging System, Rochester, NY, USA).
For statistical analysis and graph plotting, GraphPad Prism (GraphPad Software, San Diego, CA, USA) was used. Data were expressed as mean ± SE, and range where appropriate. Groups of independent data were compared using the Mann-Whitney U test. Intra-group variation was analyzed with the paired Wilcoxon test. Correlation coefficients between different analyses were calculated using the Spearman rank-test. P < 0.05 was considered statistically significant.
Baseline DAI or HBI scores did not differ significantly between steroid responding versus non-responding patients and did not predict clinical outcome. On d 28, 45% (10/22) patients with UC and 33% (8/24) patients with CD were in remission (DAI ≤ 2 and HBI ≤ 4, respectively) (Table 2, Table 3). In 26.08% (12/46) patients (5 UC, 7 CD), colectomy was carried out on d 2-26 due to steroid-refractory severe disease. The DAI and HBI scores in this subgroup did not differ significantly on d 1 between subsequently operated patients and those responding to treatment (DAI: 9.5 ± 0.7 vs 11.2 ± 0.5 for responding and operated UC patients; and HBI: 12.8 ± 2.3 vs 16.9 ± 2.3 for responding and operated CD patients) (Table 2, Table 3).
| t/d | All | Responder | Non-responder | Operated( |
| (n = 22) | (n = 10) | (n = 12) | n = 5) | |
| 1 | 9.4 (5-12) | 9.5 (5-12) | 9.4 (5-12) | 11.2 (10-12) |
| 3 | 7.4 (2-12) | 7.1 (2-10) | 7.6 (5-12) | 12 |
| 7 | 5.7 (2-9) | 4.8 (2-7) | 7.2 (5-9) | NA |
| 28 | 2.5 (0-8) | 0.8 (0-2) | 5 (3-8) | NA |
| t/d | All | Responder | Non-responder | Operated |
| (n = 24) | (n = 8) | (n = 16) | (n = 7) | |
| 1 | 14.9 (4 - 29) | 12.8 (4 - 26) | 15.9 (9 - 29) | 16.9 (11 - 29) |
| 3 | 11.2 (2 - 22) | 7.9 (2 - 13) | 12.9 (2 - 22) | 16.2 (11 - 22) |
| 7 | 7.7 (1 - 19) | 5.8 (1 - 19) | 9.6 (2 - 13) | 11.5 (10 - 13) |
| 28 | 4.2 (1 - 9) | 1.9 (1 - 3) | 6.9 (4 - 9) | NA |
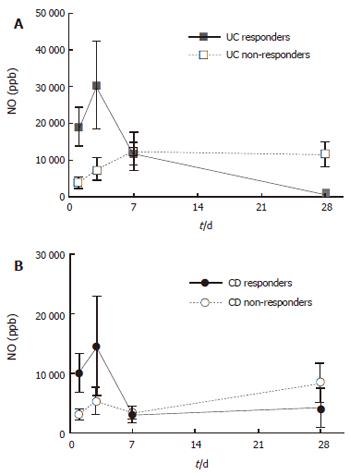
On d 1, patients with active UC and CD had greatly increased rectal NO levels (10 950 ± 7610 and 5040 ± 1280 ppb, respectively) as compared with the controls (154 ± 71 ppb, all P < 0.001). Repeat measurements of rectal NO showed a numerical increase on d 3 compared to d 1, after which a decrease was seen (Figure 1). This pattern of NO release was mainly attributed to the patients responding to steroid treatment, whereas non-responders displayed a less prominent increase on day 3, and showed no subsequent decrease (Figure 1). For steroid-responding patients, rectal NO levels decreased significantly between d 1 and 28 (from 18 860 ± 5390 to 850 ± 450 ppb in UC, and 10 060 ± 3200 to 4130 ± 3380 ppb in CD, P < 0.001 and P < 0.05, respectively).
Rectal NO levels were correlated weakly with clinical activity scores of the whole study population, DAI for UC (r = 0.34, P < 0.01), and HBI for CD (r = 0.48, P < 0.01). However, the association was clear-cut in the group of responders to treatment (r = 0.72 for UC and r = 0.64 for CD, all P < 0.001).
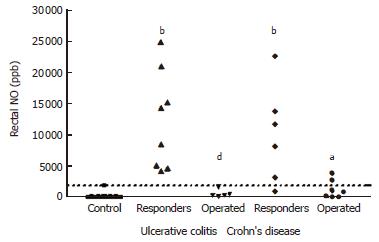
A different rectal NO pattern was seen in the subgroup of patients in whom colectomy was carried out. The rectal NO levels of these patients were significantly lower at baseline (620 ± 270 ppb in UC, and 1260 ± 550 ppb in CD) than corresponding values in the patients with a treatment response (P < 0.001 for UC and P < 0.05 for CD) (Figure 2). Applying a cut-off level of rectal NO at 2000 ppb in combination with DAI ≥ 10 for patients with UC identified all five patients with a steroid-refractory disease, leading to colectomy within 7 d, Whereas none of the UC patients responding to treatment combined rectal NO ≤ 2000 ppb and DAI ≥ 10. For CD patients, the same cut-off of NO ≤ 2000 ppb in combination with HBI ≥ 10 detected 5 of 7 patients subsequently operated, and was seen in 3 CD patients not leading to surgery.
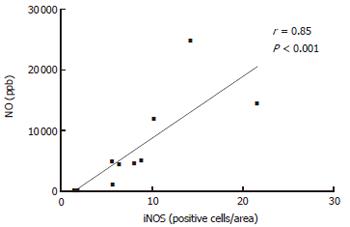
The number of iNOS-expressing cells, as judged by immunohistochemistry, was significantly higher in patients with UC and CD on d 1 as compared with the healthy controls (all P < 0.001). Furthermore, the number of iNOS-positive cells decreased between d 1 and 28 (Figure 4), reaching a borderline significance by pooling UC and CD patients (P = 0.064). The number of iNOS-positive cells was obviously correlated with rectal NO levels in patients with UC (r = 0.53, P < 0.01). This association was strengthened by analyzing the patients responding to treatment (r = 0.85, P < 0.01) (Figure 3).
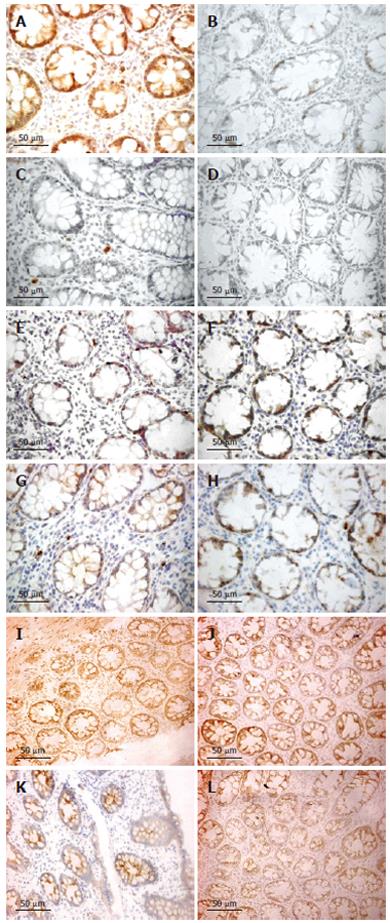
However, no correlation was found between number of iNOS-positive cells and rectal NO levels in patients with CD. The majority of iNOS-positive cells in lamina propria had the morphology of polymorphonuclear leukocytes (Figure 4).
Semi-quantitative RT-PCR showed over-expression of iNOS mRNA in the inflamed compared to normal parts of the mucosa (P < 0.05) (Figure 5). Presence of eNOS-positive cells was abundant in all sections and localized to the epithelium, but showed no temporal relationship with rectal NO levels. Recovery of nNOS staining was slight and, when occurring, mostly seen in the submucosa in nerve cells and randomly in mononuclear cells of the lamina propria.
TNF-α, IL-1β and INF-γ expressions, as judged by immunohistochemistry, were restricted to mononuclear cells in the lamina propria. IL-1β expression was significantly increased on d 1 compared to healthy controls (P < 0.05), whereas TNF-α and INF-γ were not. However, both TNF-α and IL-1β decreased numerically between d 1 and 28 for UC and CD (Figure 4). By pooling data from UC and CD patients, a significant reduction was seen (P < 0.05 for both TNF-α and IL-1β immunoreactivity). INF-γ did not change between d 1 and 28. A marked increase of iNOS-expressing cells was correlated with TNF-α expression in both UC (r = 0.46, P < 0.05) and CD (r = 0.44, P < 0.05). By pooling data for UC and CD, a weak correlation was detected for IL-1β (r = 0.33, P < 0.05). No correlation was seen between the numbers of iNOS and INF-γ-expressing cells.
This study provide evidence suggesting that the greatly increased rectal NO levels seen in active UC and CD are elaborated by high iNOS activity. In our study, a strong correlation was seen between rectal NO levels and clinical activity indices in patients responding to steroid treatment. A relationship between ongoing inflammatory activity and increased NO production in the gut is well established[6,8]. In agreement with this, we found a correlation with clinical disease activity indices in both UC and CD.
Taking into account the relatively low number of patients recruited, the subgroup analysis have to be interpretated with caution. In the non-responding group, no correlation was seen between rectal NO and clinical activity indices. This is in part explained by the aberrant rectal NO pattern in the 12 patients who subsequently underwent colectomy due to severe disease. The finding that baseline rectal NO ≤ 2000 ppb in severe UC detected all patients in need of surgery within 7 d due to steroid-refractory disease makes it tempting to suggest rectal NO measurement as a predictive marker of the need of colectomy in active UC. In CD patients, rectal NO seems to be a less strong marker for steroid-response, which is in agreement with previous studies demonstrating a 70% endoscopic remission post-treatment in UC as compared with only 13% endoscopic remission in CD[22,23], as well as a recent study by Costa et al[24] showing that fecal calprotectin is a stronger marker for relapse in UC than in CD[24].
Due to the high affinity of NO to hemoglobin[25], one may argue that luminal NO is scavenged by blood in the colon, which should cause falsely low rectal NO levels in the most severe UC and CD cases. This is however unlikely since other severe cases in our group displayed high rectal NO levels in the presence of overt rectal bleeding.
The role of NO in intestinal inflammation is unclear, as both pro-inflammatory and tissue-protective properties have been demonstrated[26]. Our observation that high rectal NO levels at the first visit was associated with a favorable clinical outcome is consistent with the idea that NO may act as an endogenous inhibitor of an aggregated immune response. Tissue-protective properties of NO have been shown in animal models of colitis[27]. The finding that NO production in collagenous colitis, a chronic inflammatory bowel disease without mucosal cell damage[28], might be even greater than in active IBD supports the concept of NO acting as a modulator of inflammatory activity in the gut. An uncontrolled chronic inflammation could, however, lead to an intracellular depletion of L-arginine, during which NOS will produce O2- instead of NO, O2- will then immediately react with NO to form ONOO-, considered to be highly cytotoxic[29].
Genetic polymorphisms of iNOS might be a plausible explanation for the different rectal NO patterns seen in the group of responding versus non-responding patients. iNOS polymorphism has previously been associated with outcome variables in different diagnoses[30-32].
There is substantial evidence pointing towards iNOS-expressing epithelial cells lining the mucosa as a major site of NO production in intestinal inflammation[13-15]. We could not confirm this finding in our present study as we encountered unspecific staining of the epithelial cells, but we consider the correlation between the number of iNOS-positive cells in lamina propria and the production of NO, measured as rectal NO levels, as circumstantial evidence, suggesting the importance of these cells in the production of the increased NO levels seen in active UC and CD. This is supported not only by earlier studies showing iNOS-positive macrophages and granulocytes in the mucosa in IBD[11], but also by our present finding of a molecular activation of iNOS within the tissue. Our morphological examination of iNOS-positive cells ascribes polymorphonuclear leukocytes as a plausible cellular source of NO production.
Whether the constitutive forms of NOS contribute to the increased NO production in IBD is unclear. For eNOS or nNOS, we could not detect any differences in staining between healthy controls, active IBD or IBD in remission. Little information is available on possible changes in eNOS and nNOS in IBD in literature. One previous study reports findings in line with our results[14], i.e. activation of mainly iNOS, whereas another group claims specific changes in eNOS and nNOS expression in UC[12].
Determination of tissue cytokine levels in IBD has resulted in disparate results. In general, pro-inflammatory cytokines (TNF-α, IL-1β and INF-γ) are believed to have a regulatory function in the activated immune response in IBD. Clearly the last years’ development has pin-pointed the key role of TNF-α in CD[3,21,33]. In line with some previous studies, we failed to show increased TNF-α expression in IBD compared to controls[34,35]. However, we found a decrease in TNF-α expression as a response to treatment, supporting data demonstrating an association between TNF-α expression and active IBD[3,36,37]. Our data showing increased IL-1β expression in active IBD compared to healthy controls are consistent with earlier studies providing evidence that IL-1β has a central role in the mucosal inflammation as seen in IBD[34,36,37]. In our study, we found no support for INF-γ as a marker of disease activity in IBD.
We found a correlation between TNF-α and IL-1β expression and iNOS expression, supporting the concept that these cytokines may have a role in inducing NO production[15].
In summary, our study shows that active UC and CD are associated with highly increased rectal NO levels. Rectal NO levels decreases in response to steroid treatment, hence offering a plausible and feasible objective method to monitor disease activity in IBD. Low rectal NO levels ≤ 2000 ppb might be a predictive marker for steroid-refractory IBD requiring acute colectomy. Furthermore, our data suggest that the major part of the increased NO production seen in active IBD is elaborated by the inducible form of NOS.
| 1. | Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986;27:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 257] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, Groessner B, Lochs H, Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999;353:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 545] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 5. | Lundberg JO, Hellström PM, Fagerhol MK, Weitzberg E, Roseth AG. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Lundberg JO, Hellström PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994;344:1673-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 185] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Herulf M, Ljung T, Hellström PM, Weitzberg E, Lundberg JO. Increased luminal nitric oxide in inflammatory bowel disease as shown with a novel minimally invasive method. Scand J Gastroenterol. 1998;33:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 315] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Ikeda I, Kasajima T, Ishiyama S, Shimojo T, Takeo Y, Nishikawa T, Kameoka S, Hiroe M, Mitsunaga A. Distribution of inducible nitric oxide synthase in ulcerative colitis. Am J Gastroenterol. 1997;92:1339-1341. [PubMed] |
| 12. | Vento P, Kiviluoto T, Järvinen HJ, Soinila S. Changes in distribution of three isoforms of nitric oxide synthase in ulcerative colitis. Scand J Gastroenterol. 2001;36:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Godkin AJ, De Belder AJ, Villa L, Wong A, Beesley JE, Kane SP, Martin JF. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996;26:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Dijkstra G, Moshage H, van Dullemen HM, de Jager-Krikken A, Tiebosch AT, Kleibeuker JH, Jansen PL, van Goor H. Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J Pathol. 1998;186:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Kolios G, Rooney N, Murphy CT, Robertson DA, Westwick J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 1998;43:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249-266. [PubMed] |
| 17. | Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894-1898. [PubMed] |
| 18. | Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2247] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 20. | Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 332] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Li CY, Ziesmer SC, Lazcano-Villareal O. Use of azide and hydrogen peroxide as an inhibitor for endogenous peroxidase in the immunoperoxidase method. J Histochem Cytochem. 1987;35:1457-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 477] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 23. | Modigliani R. Endoscopic management of inflammatory bowel disease. Am J Gastroenterol. 1994;89:S53-S65. [PubMed] |
| 24. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 426] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 25. | Wennmalm A, Benthin G, Petersson AS. Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. Br J Pharmacol. 1992;106:507-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 414] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 27. | McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 28. | Reinders CI, Herulf M, Ljung T, Hollenberg J, Weitzberg E, Lundberg JO, Hellström PM. Rectal mucosal nitric oxide in differentiation of inflammatory bowel disease and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770-6774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 549] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Tatemichi M, Sawa T, Gilibert I, Tazawa H, Katoh T, Ohshima H. Increased risk of intestinal type of gastric adenocarcinoma in Japanese women associated with long forms of CCTTT pentanucleotide repeat in the inducible nitric oxide synthase promoter. Cancer Lett. 2005;217:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Shen J, Wang RT, Wang LW, Xu YC, Wang XR. A novel genetic polymorphism of inducible nitric oxide synthase is associated with an increased risk of gastric cancer. World J Gastroenterol. 2004;10:3278-3283. [PubMed] |
| 32. | Gonzalez-Gay MA, Llorca J, Sanchez E, Lopez-Nevot MA, Amoli MM, Garcia-Porrua C, Ollier WE, Martin J. Inducible but not endothelial nitric oxide synthase polymorphism is associated with susceptibility to rheumatoid arthritis in northwest Spain. Rheumatology (Oxford). 2004;43:1182-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2287] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 34. | Stevens C, Walz G, Singaram C, Lipman ML, Zanker B, Muggia A, Antonioli D, Peppercorn MA, Strom TB. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Gotteland M, Lopez M, Muñoz C, Saez R, Altshiller H, Llorens P, Brunser O. Local and systemic liberation of proinflammatory cytokines in ulcerative colitis. Dig Dis Sci. 1999;44:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Woywodt A, Ludwig D, Neustock P, Kruse A, Schwarting K, Jantschek G, Kirchner H, Stange EF. Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Carty E, De Brabander M, Feakins RM, Rampton DS. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 2000;46:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
S- Editor Pan BR L- Editor Kumar M E- Editor Ma WH