Published online May 28, 2006. doi: 10.3748/wjg.v12.i20.3196
Revised: April 7, 2006
Accepted: April 16, 2006
Published online: May 28, 2006
The prevalence of obesity in the United States has reached epidemic proportions. With more than 30 million Americans clinically obese, the younger population has also been affected. Surgical therapy should be offered to the severely obese patient who is refractory to nonsurgical therapy, as established by the 1991 NIH Consensus Conference on Gastrointestinal Surgery for Severe Obesity. Surgery is currently the most effective therapy for weight loss. It is far more effective than any other treatment modality, both in terms of the amount of weight loss and in terms of durability in maintaining weight loss.
- Citation: Eisenberg D, Duffy AJ, Bell RL. Update on obesity surgery. World J Gastroenterol 2006; 12(20): 3196-3203
- URL: https://www.wjgnet.com/1007-9327/full/v12/i20/3196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i20.3196
The prevalence of obesity in the United States has reached epidemic proportions[1]. With more than 30 million Americans clinically obese, the younger population has also been affected. The number of obese young adults between the ages of 18 and 29 has increased by nearly 50% in the 1990’s[2-4], and currently, nearly 25% of children are believed to be obese[5]. Obesity now represents a staggering 5% of the total health care costs in the United States[6]. This, however, is not only an American problem. The prevalence of obesity worldwide is also increasing. In England, the number of obese men and women has tripled over the twenty years between 1980 and 2000[7]. More than 1.5 billion people worldwide are thought to be obese or overweight[8]. Obesity, clinically defined as a body mass index (BMI) greater than 30 kg/m2, is associated with comorbid conditions affecting nearly every organ system, including the cardiovascular, endocrine, renal, pulmonary, gastrointestinal, and musculoskeletal systems. Type 2 diabetes, hypertension, hyperlipidemia, obstructive sleep apnea, stroke, heart disease, renal disease, degenerative joint disease, venous disease and several forms of cancer have all been associated with obesity[9,10]. In addition, the obese are frequently afflicted by depression, anxiety and low self-esteem[11]. In the United States, obesity leads to a significant decrease in life expectancy and an annual cost of approximately $100 billion, including nearly $4 billion in lost productivity[12,13]. In severely obese individuals (BM I> 35 kg/m2), the mortality is 12 times higher in men aged 25-34 years and 6 times higher in men aged 35-44 years compared to age-matched nonobese control subjects[14].
In severely obese people, diet, exercise, and pharmacotherapy can produce a modest weight loss in the range of 2%-10% at 1 year. Unfortunately weight regain is common. The worsening obesity crisis, combined with the lack of a durable medical treatment option, has led to an increased interest in surgical approaches for the treatment of obesity. Bariatric surgery may be broadly defined as surgical treatment strategies designed to limit caloric intake, decrease nutrient absorption, or both. Procedures designed to limit caloric intake, termed restrictive procedures, include gastric banding or the placement of intragastric balloons. Surgical procedures that decrease nutrient absorption are termed malabsorptive procedures. Jejunal-ileal bypass, a now historical operation, can be considered a pure malabsorpitve procedure. The most commonly performed bariatric operations, such as gastric bypass and biliopancreatic diversion, introduce elements of caloric restriction and partial nutrient malabsorption[15]. Worldwide, the gastric bypass is the single most frequently performed operation for obesity[16], with more than half being done laparoscopically[17]. Most importantly, patients who undergo gastric bypass have been able to achieve long-term excess weight loss in the range of 49%-62%[18,19,20].
For severely obese adults with weight-related comorbidities, surgery provides the most effective and durable approach for clinically significant weight loss. There is yet no consensus about surgery for severely obese adolescents, but it is nonetheless gaining in popularity, with demonstration of significant and durable weight loss in individuals 12 to 18 years old[5].
Overweight and obesity are independent risk factors for increased mortality. Dramatic increase in mortality is noted when BMI exceeds 30 kg/m2. This risk further increases the longer the duration of obesity[23,24]. Disappointingly, only 27% to 42% of obese patients are advised by their healthcare professional to lose weight[21,22]. A variety of non-surgical options are available for the treatment of obesity, including exercise therapy, behavioral and dietary modification, and pharmacotherapy[25]. Successful long-term weight loss may be defined as maintaining a 10% weight-loss over a period of one year, or more strictly as an average weight loss of 30 kg for an average of 5.5 years[26]. For the majority of obese individuals, sufficient weight loss is infrequently obtained by conventional methods.
Dietary modification and nutritional counseling is an essential first step in the treatment of obesity. Unfortunately, no dietary approach has ever demonstrated sustained weight reduction in the severely obese population[6]. Recently, a randomized trial compared common diets for efficacy of weight loss[28]. The Atkins, Ornish, Weight Watchers and Zone diets were compared in patients with a mean BMI of 35 kg/m2. None of the diets studied produced a mean weight loss of more than 10 kg at one year. Behavioral therapy in combination with dietary modification appears to be more effective than either method alone, but most patients trend towards their pre-treatment weight within 5 years of treatment[29]. Physical activity, as a method for weight reduction, is a necessary component of most weight-loss programs. Consistent exercise has been shown to effect longer lasting weight loss, and the level of physical activity directly correlates with the degree of weight loss[30]. Diet with exercise produces superior results compared to either diet or exercise alone. At one-year follow-up, the addition of exercise to diet alone appears to give more stable weight loss. However, the total weight loss still remains modest, in the range of 6-9 kg after 1 year[31].
Appetite suppressants are also used to effect weight loss. These include serotonin selective re-uptake inhibitors (SSRI’s), serotonergic, and noradrenergic agents. Such anorectic agents are usually used in combination with a designed low-calorie diet. With one exception, all studies evaluating their efficacy have shown that a combination of diet and appetite suppressants appear to be effective during the first 6 mo of treatment, but fail to show long-term effectiveness beyond 12 mo[25,32]. Orlistat, a newer agent that induces fat malabsorption, similarly will only lead to an initial excess weight loss of 10%[6]. Long-term studies are less optimistic. After 52 wk of three times per day therapy, when combined with a low calorie diet, orlistat will only lead to a weight loss of 5%[27].
Surgical therapy should be offered to the severely obese patient that is refractory to nonsurgical therapy, as established by the 1991 NIH Consesus Conference on Gastrointestinal Surgery for Severe Obesity[33]. The impetus for the introduction of surgical therapy for obesity was borne from the high incidence of long-term weight regain in the severely obese. Patients are eligible for bariatric surgery if they have failed nonsurgical treatments and have a BMI > 40 kg/m2 or BMI > 35 kg/m2 with comorbidities.
The obese patient undergoing abdominal surgery warrants special consideration in the preoperative workup. These patients have an overall higher mortality compared with nonobese patients when undergoing gastrointestinal surgery (6.6% vs 2.6%)[34]. As they carry higher risks for cardiovascular disease, pulmonary and thromboembolic complications, and wound infections, special care must be taken in preparing these patients for surgery[35]. Operative weight has been shown to directly correlate with an increase in cardiovascular events in both men and women[36]. Risk stratification and appropriate preoperative intervention, therefore, is important in this patient population. Low-risk patients should receive perioperative beta-blockade, while patients that are intermediate and high-risk should undergo noninvasive cardiac testing preoperatively[35].
The risk for deep venous thrombosis and pulmonary embolism is also higher in the obese population compared with the general population. Specific comorbidities in these patients have been identified that increase the risk of deep venous thrombosis (DVT) or pulmonary embolism (PE). These include the presence of venous stasis disease, BMI > 60 kg/m2, truncal obesity, and obstructive sleep apnea[37]. The presence of clinically severe obesity alone is associated with a high rate of pulmonary embolism in postoperative autopsies[38]. The preoperative use of 5000 units of subcutaneous unfractionated heparin, and every 8 h thereafter during the hospitalization, in conjunction with sequential compression devices, has been shown to provide adequate DVT prophylaxis[39]. Alternatively, low-molecular weight heparin may be used for DVT prophylaxis[40,35]. In patients with a very high risk for postoperative DVT/PE, the preoperative placement of an inferior vena cava (IVC) filter should be considered[41].
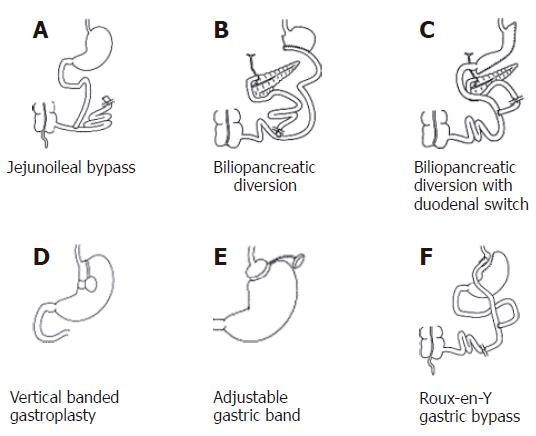
The field of obesity surgery began in the early 1950’s with the jejunoileal bypass, a malabsorptive procedure in the strictest of definitions[42]. As its name implies, this procedure bypasses a large segment of small intestine by connecting the proximal jejunum (roughly 14 inches from the ligament of Treitz) to distal ileum, about 4 inches proximal to the ileocecal valve (Figure 1A). Weight loss is produced by malabsorption of macronutrients, namely carbohydrates, fats, and proteins[17]. Severe diarrhea and nutritional deficiencies frequently result, leading to significant metabolic abnormalities, nephrolithiasis, cholelithiasis, osteopenia, and hepatic cirrhosis. In the past, metabolic bone disease and vitamin deficiencies often necessitated reversal of the procedure[43]. With time, other procedures carried a significantly lower complication profile and thus supplanted the jejunoileal bypass[44]. This procedure is now of historical significance only.
The vast majority of complications that resulted from jejunoileal bypass resulted from the very long “blind” limb of intestine that neither biliopancreatic juices nor victuals flowed through. Newer malabsorptive procedures have eliminated this “blind” limb[18]. The biliopancreatic diversion (BPD), first described by Scopinaro in 1979[45], involves the creation of a distal gastrectomy with a Roux-Y reconstruction using a 250-cm alimentary tract limb as measured from the ileocecal junction (Figure 1B). The long biliopancreatic limb is anastomosed to the distal ileum, creating a short (50-cm) common channel for nutrient absorption[47]. Weight loss occurs because the ingested food is not entirely digested and absorbed. This is a true malabsorptive procedure. In contrast to purely restrictive procedures, BPD allows patients to continue eating “normally” while achieving significant weight loss[54]. In BPD, nutrient absorption occurs predominantly in the short distal common channel, where both the chyme and biliopancreatic juices mix.
Short-term weight-loss has been reported at almost 15% at 4 mo[47] and more importantly, in a published series of more than 2000 patients[46], a mean long-term weight reduction of 75% of the initial excess weight was achieved. Late complications include anemia, stomal ulcers, bone demineralization and neurologic dysfunction due to vitamin deficiencies. The vitamins most affected are the fat-soluble vitamins A, D, K, and E. Calcium and iron, primarily absorbed in the duodenum, can also be significantly affected. Protein calorie malnutrition has been documented, as well as hepatic failure and polyarthritis[48,49]. Due to the late onset of significant metabolic complications associated with the procedure, it has fallen out of favor in the United States[48].
The biliopancreatic diversion with duodenal switch (BPD-DS) adds a sleeve gastrectomy (instead of distal gastrectomy) and duodenoileostomy to the BPD (Figure 1C). The common channel is increased in length from 50 to 100 cm. This procedure was first performed in 1990, with several modifications in the duodenal switch portion to its current form[54]. BPD-DS was first performed laparoscopically by Gagner et al in 1999[53]. The duodenal switch modification was made to try to reduce the complication rate, while maintaining reliable weight loss. Although durable weight loss is comparable to the BPD, long-term complications, mostly intractable diarrhea, are still a major concern[50]. Micronutient supplementation is still mandatory to help minimize the risk of vitamin deficiency syndromes and osteoporosis[54]. Nonetheless it remains a very effective treatment for the superobese patients (BMI > 50 kg/m2). Excess weight loss ranges from nearly 60% to greater than 90% in 10 to 24 mo[51]. At 8 years, excess weight loss may be as high as 70%[52]. In experienced hands, this procedure can be performed safely via the laparoscopic approach[53].
The vertical banded gastroplasty (VBG) was first described by Mason in 1982[55]. This procedure is a purely restrictive procedure; it leaves the rest of the gastrointestinal tract intact for nutrient absorption. In this procedure, a full thickness hole is made with a circular stapling device in the proximal stomach. Using this hole, the proximal stomach is stapled, parallel to the lesser curve, up to the angle of His. This creates a pouch of approximately 50 mL capacity (Figure 1D). A band of silastic or polypropylene mesh is commonly placed through the hole, circumferentially around the distal portion of the tubularized pouch, thereby limiting the stomal outlet diameter to roughly 1 cm[48,55].
Vertical banded gastroplasty may be performed open or with laparoscopic techniques. Results of VBG vary. Excess weight loss in the first year after surgery is in the range of 60%. Patient satisfaction, meanwhile, ranges from 75% to greater than 90%[56,57]. Five-year follow-up suggests less satisfactory weight loss, with the average excess weight loss being 30%-54%. Frequently, patients cited difficulties in tolerating a regular diet[59-61]. Most disappointingly, a prospective study following patients for more than 10 years after vertical banded gastroplasty found that only 20% of patients maintained the loss of at least 50% of excess body weight[58]. Additionally, a significant portion of patients had persistent vomiting, gastroesophageal reflux, or heartburn. Furthermore, the rate of re-operation may be as high as 29%[62]. When compared to gastric bypass, multiple studies have shown the superior results of gastric bypass over VBG in terms of durable weight loss and long-term outcome. Subsequently, VBG has fallen into disfavor[43,63,64].
Adjustable gastric banding is a restrictive procedure that does not involve stapling of the gastrointestinal tract. It is considered the least invasive of the operations for severe obesity[65]. The key feature of the current gastric banding devices is the adjustability of the outer diameter of the gastric band. Thus, oral intake can be restricted if the band is tightened, or the band can be loosened in patients that present with signs or symptoms of obstruction. Dietary compliance is mandatory for the success of the adjustable gastric band. Patients can bypass the effects of the banded gastric pouch by consuming high-calorie liquid diets, thus emphasizing the need for continued cooperation postoperatively between the physician and patient.
Initially described as an open procedure by Kuzmak in 1986, an adjustable silicon band is used to encircle the proximal stomach, creating a small gastric pouch and narrowed outlet[66]. The only device currently approved in the United States is the Lap-Band (Inamed Health, Santa Barbara, California), but is one of several commercially available devices worldwide. The Lap-Band consists of a silicon adjustable elastic band that is placed around the proximal stomach via the pars flaccida, creating a pouch volume of 10-15 mL (Figure 1E). A subcutaneous port enables percutaneous needle access to adjust the volume of the gastric band and consequently the size of the gastric pouch outlet[67]. Although results in Europe have been encouraging[68], the early results in the United States were considered poor relative to other bariatric procedures, with only 36% excess weight loss after three years. Later studies showed improvement with average excess weight loss of 42% at one-year follow-up[69]. A more recent prospective study produced much more impressive results[70] with mean excess weight loss of 50% at one year, 61% at two years, and 65% at three years.
Early complications are uncommon in this laparoscopic procedure and conversion to open operation is infrequent[70,71]. Overall complication rates vary in the literature between 9%-20%[69,70], including band slippage or prolapse, dysphagia, port migration, port-site infection, tube breakage, dilatation of the gastric pouch, gastric perforation, and erosion of the band through the stomach wall[43,70-73]. Some of these complications are treated electively with laparoscopic reposition or replacement of the Lap-Band device, or with repositioning or repair of tubing or the port. Complication rates and frequency of reoperation likely reflect overall experience with the procedure and optimal band positioning. The longer European experience reflects a learning curve with decreasing morbidity and need for reoperation. Total complication rates are 9%-11% in most European studies, but 10%-20% in U.S. studies. Depending on the institution, the reoperation rate varies from 1% to 13%[43,69,70,73-75]. Malnutrition and vitamin deficiencies are uncommon.
The Swedish version of the gastric band, the Swedish Adjustable Gastric Band (AB Obtech, Sweden), was introduced in 1985 by Hallberg et al[76]. It consists of an inflatable double-silicon band reinforced by a Dacron net. The silicon balloon on the inner surface can be regulated by a subcutaneous port. Its complication profile is similar to the Lap Band, including band erosion, erosive esophagitis, pouch dilatation, disconnection of tubing, invagination of distal gastric wall, and leakage from the balloon[76,77]. Several studies showed promising results for the Swedish Band. At a five-year follow-up mean excess weight loss was 57%, with approximately 83% of patients obtaining more than 50% excess weight loss[78]. Similar results were obtained by Australian investigators[79] with a mean excess weight loss of 60% at 12 mo, and 69% of patients had a greater than 50% excess weight loss over the same follow-up.
A Swiss prospective, randomized trial compared 90 patients treated with Lap Band and 90 patients treated with the Swedish Adjustable Gastric Band (SAGB)[80]. The SAGB appeared more susceptible to infection and early complication. Although the patients with Lap Band lost more excess weight sooner, the two cohorts trended towards equal excess weight loss by 30 mo post-operatively. At three-year follow-up, only about half of each treatment group lost at least 50% of excess weight. An earlier study with a short follow-up of less than 12 mo showed no difference between the two gastric bands in terms of weight loss and complications[81]. An additional meta-analysis reviewing the two laparoscopic gastric bands revealed similar excess weight loss, although the Lap Band seemed to have a slightly higher long-term complication rate[82]. Overall there appears to be little major difference between the two banding systems.
Another, much less common technique, is the placement of an intragastric balloon. First introduced in the 1980’s, preliminary studies in pigs showed ambiguous results when an inflatable balloon was introduced into the stomach in an attempt to reduce stomach capacity and oral intake[15,83]. In humans, the intragastric balloon is inserted orally using a special application sheath. It is placed 10 cm distal to the gastroesophageal junction and inflated with approximately 500 mL of saline, attaining a diameter of 10 cm. The volume can be subsequently adjusted to 400-800 mL. Smaller, 200 mL, air-filled balloons are also available. Large hiatal hernias and peptic ulcer disease usually preclude the use of the intragastric balloon. Immediate complications include nausea, vomiting, and abdominal cramps, suggesting intolerance of the balloon[84]. Other complications include gastroesophageal reflux, belching, and esophageal erosions. As of yet, there are no long-term studies to prove the efficacy of this technique. Relative to other bariatric procedures, weight loss results are modest at best. The greatest reported results are in the range of 10% weight loss sustained at two years[84].
Gastric pacing, first introduced in humans in 1996[85], is not widely accepted and still considered experimental. It attempts to provide a non-malabsorptive and non-restrictive method for significant, durable weight loss. The purported mechanism of action is to disrupt normal gastric myoelectrical activity, which subsequently affects both gastric emptying and gastric relaxation. The implantable gastric stimulator (IGS; Transcend, Transneuronix, Inc., New Jersey) includes a battery-operated generator and a bipolar lead. Electrodes are placed into the muscle layer on the lesser curvature of the anterior gastric wall[86]. The electrode delivers a bipolar pulse through its connection to a pacer that sits in a subcutaneous pocket[42]. It is postulated that electrical stimulation disrupts eating behavior by causing a change in parasympathetic activity in the stomach, leading to gastroparesis[42,87]. Weight loss should therefore be induced by consistent and predictable early satiety. Early human studies in a small cohort showed weight loss in only half the patients[85]. Later studies, also in a small number of patients, were able to demonstrate a modest, but stable weight loss[86]. Thus far, studies on gastric pacing have been plagued by mechanical problems relating to the electrical leads.
The procedure is safely and preferably performed laparoscopically[88]. A large multicenter, randomized, controlled, double-blinded American trial is under way[89]. Early results showed a mean weight loss of 11% at one year. A second round, pilot trial is in progress, employing four electrodes and thus far, a mean excess weight loss of 21% was seen at 7-mo follow-up[89]. Although these results are modest compared to other bariatric procedures, the optimal use of the electrodes, the number of electrodes, and the direction of stimulation has yet to be determined.
The Roux-Y gastric bypass, a mixed restrictive and malabsorptive procedure, is considered the gold standard for bariatric surgery[6], and is currently the procedure of choice in the United States[16]. The gastric bypass operation was first introduced by Mason et al[90] in 1967. In the initial description of the operation, a loop gastrojejunostomy was created. The Roux-Y gastric bypass (RYGB) was subsequently introduced in 1977[44]. In this procedure, a small gastric pouch of 15-30 mL is created by transecting the proximal stomach. Intestinal continuity is reestablished via a Roux-Y gastrojejunostomy (Figure 1F). With the resultant anatomy, dietary foodstuff bypasses the distal stomach, duodenum, and proximal jejunum. Unlike the true malabsorptive procedures, protein calorie malnutrition is rare. The only malabsorption that takes place with RYGB is micronutrient malabsorption, mainly folate, calcium, iron and vitamin B12.
Gastric bypass is effective for many reasons. First, the small proximal gastric pouch restricts the food portions that the patient is able to ingest. It forces the patient into good eating behaviors such as thoroughly chewing one’s food and eating slowly. For the first several months after surgery, daily caloric intake ranges from 1674 to 3347 kJ. The second, equally important, contributor to weight loss is that the low level of caloric intake is well tolerated by patients; they do not usually feel hungry. Unlike most other bariatric procedures, gastric bypass results in a decrease in serum ghrelin levels. Ghrelin is a hormone responsible for satiety, and is produced by cells located in the gastric fundus and proximal duodenum in response to an empty stomach. When ghrelin is released into the bloodstream, it stimulates feeding behaviors. The level of ghrelin falls shortly after eating and is clearly one hormone responsible for satiety. After gastric bypass, ghrelin levels are low and remain low for an indefinite period of time, resulting in a prolonged decrease in appetite[91].
When open Roux-Y gastric bypass is compared to open vertical banded gastroplasty (VBG), patients who undergo gastric bypass have superior weight loss as well as long-term weight-loss maintenance[64,92]. Similarly, in a randomized trial of 83 patients, laparoscopic Roux-Y gastric bypass out-performed laparoscopic VBG[63]. Despite the relative simplicity of performing a VBG and, consequently, shorter operative times, weight reduction was significantly greater after laparoscopic gastric bypass at one and two years postoperatively. After gastric bypass, weight loss is significant and durable. Multiple studies support the use of the Roux-Y gastric bypass for the treatment of the severely obese. A mean of greater than 60% excess weight loss is seen at 1-, 2-, 3- and 5-years postoperatively[64,93-95]. Long-term follow-up has been reported at fourteen years, with an excess weight loss of 49%[96]. The best results, obtained by Fobi et al, show 80% excess weight loss at two years[97].
The Roux-Y gastric bypass, first described as a laparoscopic procedure in 1994 by Wittgrove et al[98], has proven to be safe and effective and has quickly gained popularity[99]. The operation is more challenging than the open gastric bypass, however, and a learning curve of approximately 100 cases should be expected[100,101]. Despite slightly longer operative times in the laparoscopic operation, patients who undergo laparoscopic RYGB have less blood loss, shortened hospitalization time, and a faster return to normal activities when compared to patients who undergo open RYGB. For laparoscopic RYGB, the average length of postoperative stay is 2 d[102-104]. A case series of 400 patients undergoing laparoscopic Roux-Y gastric bypass revealed an average excess weight loss of 69% after one year. This varied by BMI, with slightly diminished results in the heavier patients[99]. The study also showed importantly that operative times decreased steadily with operative experience. This finding is supported by other studies as well[101]. Wittgrove and Clark showed an excess weight loss of 80% after the first year[105]. Schauer et al[103] found an excess weight loss of 83% at two years and 77% at 30 mo using the laparoscopic approach.
Laparoscopic gastric bypass is also safe and effective in the super obese (BMI > 60 kg/m2). For patients with a BMI less than 60 kg/m2 the excess weight loss is greater than 60% at 1 year in multiple studies whereas in the super-obese, excess weight loss in the range of 50%. The complication profile, however, is similar through a range of BMIs[99,106,107]. Recently, laparoscopic Roux-Y gastric bypass was shown to be safe and effective in patients with a BMI > 70 kg/m2[108]. Overall, the complication rate following gastric bypass is 10%-15% and mortality is approximately 0.3%[43,101]. Complications include anastomotic leak, thromboembolic events, bleeding, anastomotic stricture, hernia, marginal ulceration, vitamin malnutrition, gallstones, and wound infection. In the laparoscopic approach, complication rates sharply decrease along the surgeon’s progress on the operative learning curve[101]. Wound infection and incisional hernia, the most common complication in the open operation, are found at a rate of < 5% and < 1%, respectively, in laparoscopic Roux-Y gastric bypass. Late anastomotic stricture, however, was found more commonly with the laparoscopic approach, nearing 10% in some series[101-103,109]. Nutrient and vitamin deficiencies created by this procedure are easily correctable and, more importantly, preventable with appropriate supplementation. Due to the outstanding weight loss that can be achieved with a relatively low complication profile, laparoscopic Roux-Y gastric bypass has become the bariatric procedure of choice.
Bariatric procedures in general, and the gastric bypass in particular, produce dramatic improvements of comorbidities associated with severe obesity, with documented resolution of type 2 diabetes and glucose intolerance. Although the exact mechanism is unknown, it appears that the gastrointestinal bypass procedure itself, not just the actual weight loss, plays the major physiologic role in these findings[110]. In addition, improvement or resolution was seen postoperatively in patients with other components of the metabolic syndrome such as hypertension and dyslipidemia. In a comparison of 114 case series, 64%-100% of postoperative patients showed improvement or resolution of their diabetes. A study of 11 reports showed an improvement or resolution of dyslipidemia in 60%-100% of patients. In 19 studies, hypertension showed an improvement or resolution in 25%-100% of patients[17]. Two years after obesity surgery, the incidence of hypertension, diabetes, and lipid abnormalities are reduced relative to medically treated patients. Followed to eight years, the incidence of diabetes showed the most durable reduction, while the reduction in dyslipidemia and hypertension was more modest[112,113]. With appropriate weight reduction, dramatic improvements in sleep apnea are also seen[17,111].
In conclusion, surgery is currently the most effective therapy for weight loss. It is far more effective than any other treatment modality, both in terms of the amount of weight loss and in terms of durability in maintaining weight loss. Additionally, the surgical treatment of obesity produces results that greatly improve or reverse the comorbid conditions often associated with severe obesity. These include diabetes, hypertension, dyslipidemia, and obstructive sleep apnea. This is most apparent in patients who had undergone a Roux-Y gastric bypass. The search for a less invasive, equally effective weight loss modality is still underway. Ideally a non-operative medical therapy will emerge that will produce equal or near-equal results. Until then, surgery will remain the treatment of choice for this population of patients.
| 1. | Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 694] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1359] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 3. | Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1369] [Cited by in RCA: 1202] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 4. | Greenway SE, Greenway FL 3rd, Klein S. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg. 2002;137:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102-107; discussion 107-108;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Demaria EJ, Jamal MK. Surgical options for obesity. Gastroenterol Clin North Am. 2005;34:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, Smith WC, Jung RT, Campbell MK, Grant AM. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii-iv, 1-182. [PubMed] |
| 8. | Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 2902] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 10. | Agnani S, Vachharajani VT, Gupta R, Atray NK, Vachharajani TJ. Does treating obesity stabilize chronic kidney disease. BMC Nephrol. 2005;6:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | van Hout GC, van Oudheusden I, van Heck GL. Psychological profile of the morbidly obese. Obes Surg. 2004;14:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1546] [Cited by in RCA: 1453] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 13. | Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 640] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 14. | Drenick EJ, Bale GS, Seltzer F, Johnson DG. Excessive mortality and causes of death in morbidly obese men. JAMA. 1980;243:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 171] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Del Castillo Déjardin D, Sabench Pereferrer F, Hernández González M, Blanco Blasco S, Abelló Sala M. The evolution of experimental surgery in the field of morbid obesity. Obes Surg. 2004;14:1263-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 444] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 17. | Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 952] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 18. | Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S-16S. [PubMed] |
| 19. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4763] [Article Influence: 216.5] [Reference Citation Analysis (2)] |
| 20. | White S, Brooks E, Jurikova L, Stubbs RS. Long-term outcomes after gastric bypass. Obes Surg. 2005;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Flocke SA, Clark A, Schlessman K, Pomiecko G. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421. [PubMed] |
| 22. | Galuska DA, Will JC, Serdula MK, Ford ES. Are health care professionals advising obese patients to lose weight. JAMA. 1999;282:1576-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 417] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 23. | Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1156] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 24. | Orzano AJ, Scott JG. Diagnosis and treatment of obesity in adults: an applied evidence-based review. J Am Board Fam Pract. 2004;17:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Douketis JD, Feightner JW, Attia J, Feldman WF. Periodic health examination, 1999 update: 1. Detection, prevention and treatment of obesity. Canadian Task Force on Preventive Health Care. CMAJ. 1999;160:513-525. [PubMed] |
| 26. | Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 924] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 27. | Berne C. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabet Med. 2005;22:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1210] [Cited by in RCA: 1054] [Article Influence: 50.2] [Reference Citation Analysis (11)] |
| 29. | Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13 Suppl 2:39-46. [PubMed] |
| 30. | Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome. Am J Clin Nutr. 2003;78:684-689. [PubMed] |
| 31. | Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Weintraub M. Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study. Clin Pharmacol Ther. 1992;51:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55:615S-619S. [PubMed] |
| 34. | Postlethwait RW, Johnson WD. Complications following surgery for duodenal ulcer in obese patients. Arch Surg. 1972;105:438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 84] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Abir F, Bell R. Assessment and management of the obese patient. Crit Care Med. 2004;32:S87-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Kannel WB, D'Agostino RB, Cobb JL. Effect of weight on cardiovascular disease. Am J Clin Nutr. 1996;63:419S-422S. [PubMed] |
| 37. | Sapala JA, Wood MH, Schuhknecht MP, Sapala MA. Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg. 2003;13:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Melinek J, Livingston E, Cortina G, Fishbein MC. Autopsy findings following gastric bypass surgery for morbid obesity. Arch Pathol Lab Med. 2002;126:1091-1095. [PubMed] |
| 39. | Miller MT, Rovito PF. An approach to venous thromboembolism prophylaxis in laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Wu EC, Barba CA. Current practices in the prophylaxis of venous thromboembolism in bariatric surgery. Obes Surg. 2000;10:7-13; discussion 14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Ferrell A, Byrne TK, Robison JG. Placement of inferior vena cava filters in bariatric surgical patients--possible indications and technical considerations. Obes Surg. 2004;14:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950-2000. Obes Surg. 2002;12:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Schneider BE, Mun EC. Surgical management of morbid obesity. Diabetes Care. 2005;28:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Griffen WO Jr, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 262] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Lombezzi R, Friedman D, Bachi V. [The bilio-pancreatic bypass for functional surgical treatment of obesity]. Minerva Med. 1979;70:3537-3547. [PubMed] |
| 46. | Scopinaro N, Adami GF, Marinari GM, Gianetta E, Traverso E, Friedman D, Camerini G, Baschieri G, Simonelli A. Biliopancreatic diversion. World J Surg. 1998;22:936-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 367] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | de Luis DA, Pacheco D, Izaola O, Romero A, Marcos JL, Pelaz J, Barrera A, Cabezas G, Terroba MC, Cuellar L. Early clinical and surgical results of biliopancreatic diversion. Obes Surg. 2005;15:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Livingston EH. Obesity and its surgical management. Am J Surg. 2002;184:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Grimm IS, Schindler W, Haluszka O. Steatohepatitis and fatal hepatic failure after biliopancreatic diversion. Am J Gastroenterol. 1992;87:775-779. [PubMed] |
| 50. | Murr MM, Balsiger BM, Kennedy FP, Mai JL, Sarr MG. Malabsorptive procedures for severe obesity: comparison of pancreaticobiliary bypass and very very long limb Roux-en-Y gastric bypass. J Gastrointest Surg. 1999;3:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Gagner M, Matteotti R. Laparoscopic biliopancreatic diversion with duodenal switch. Surg Clin North Am. 2005;85:141-149, x-xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 483] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 53. | Ren CJ, Patterson E, Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes Surg. 2000;10:514-523; discussion 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 309] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, Biron S. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 386] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 55. | Mason EE. Vertical banded gastroplasty for obesity. Arch Surg. 1982;117:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 316] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Kalfarentzos F, Kechagias I, Soulikia K, Loukidi A, Mead N. Weight loss following vertical banded gastroplasty: intermediate results of a prospective study. Obes Surg. 2001;11:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Choi Y, Frizzi J, Foley A, Harkabus M. Patient satisfaction and results of vertical banded gastroplasty and gastric bypass. Obes Surg. 1999;9:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Balsiger BM, Poggio JL, Mai J, Kelly KA, Sarr MG. Ten and more years after vertical banded gastroplasty as primary operation for morbid obesity. J Gastrointest Surg. 2000;4:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Baltasar A, Bou R, Arlandis F, Martínez R, Serra C, Bengochea M, Miró J. Vertical banded gastroplasty at more than 5 years. Obes Surg. 1998;8:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | MacLean LD, Rhode BM, Forse RA. Late results of vertical banded gastroplasty for morbid and super obesity. Surgery. 1990;107:20-27. [PubMed] |
| 61. | Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 310] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Suter M, Jayet C, Jayet A. Vertical banded gastroplasty: long-term results comparing three different techniques. Obes Surg. 2000;10:41-46; discussion 47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Olbers T, Fagevik-Olsén M, Maleckas A, Lönroth H. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic vertical banded gastroplasty for obesity. Br J Surg. 2005;92:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Sugerman HJ, Londrey GL, Kellum JM, Wolf L, Liszka T, Engle KM, Birkenhauer R, Starkey JV. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 173] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Belachew M, Legrand M, Vincent V, Lismonde M, Le Docte N, Deschamps V. Laparoscopic adjustable gastric banding. World J Surg. 1998;22:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Kuzmak LI. A Review of Seven Years' Experience with Silicone Gastric Banding. Obes Surg. 1991;1:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Ren CJ, Fielding GA. Laparoscopic adjustable gastric banding: surgical technique. J Laparoendosc Adv Surg Tech A. 2003;13:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Angrisani L, Di Lorenzo N, Favretti F, Furbetta F, Iuppa A, Doldi SB, Paganelli M, Basso N, Lucchese M, Zappa M. The Italian Group for LAP-BAND: predictive value of initial body mass index for weight loss after 5 years of follow-up. Surg Endosc. 2004;18:1524-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Ren CJ, Horgan S, Ponce J. US experience with the LAP-BAND system. Am J Surg. 2002;184:46S-50S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Holloway JA, Forney GA, Gould DE. The Lap-Band is an effective tool for weight loss even in the United States. Am J Surg. 2004;188:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | O'Brien PE, Dixon JB, Brown W, Schachter LM, Chapman L, Burn AJ, Dixon ME, Scheinkestel C, Halket C, Sutherland LJ. The laparoscopic adjustable gastric band (Lap-Band): a prospective study of medium-term effects on weight, health and quality of life. Obes Surg. 2002;12:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Niville E, Dams A, Van Der Speeten K, Verhelst H. Results of lap rebanding procedures after Lap-Band removal for band erosion -- a mid-term evaluation. Obes Surg. 2005;15:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Favretti F, Cadière GB, Segato G, Himpens J, De Luca M, Busetto L, De Marchi F, Foletto M, Caniato D, Lise M. Laparoscopic banding: selection and technique in 830 patients. Obes Surg. 2002;12:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | O'Brien PE, Dixon JB. Weight loss and early and late complications--the international experience. Am J Surg. 2002;184:42S-45S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Angrisani L, Furbetta F, Doldi SB, Basso N, Lucchese M, Giacomelli F, Zappa M, Di Cosmo L, Veneziani A, Turicchia GU. Lap Band adjustable gastric banding system: the Italian experience with 1863 patients operated on 6 years. Surg Endosc. 2003;17:409-412. [PubMed] |
| 76. | Hauri P, Steffen R, Ricklin T, Riedtmann HJ, Sendi P, Horber FF. Treatment of morbid obesity with the Swedish adjustable gastric band (SAGB): complication rate during a 12-month follow-up period. Surgery. 2000;127:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Westling A, Bjurling K, Ohrvall M, Gustavsson S. Silicone-adjustable gastric banding: disappointing results. Obes Surg. 1998;8:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Steffen R, Biertho L, Ricklin T, Piec G, Horber FF. Laparoscopic Swedish adjustable gastric banding: a five-year prospective study. Obes Surg. 2003;13:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Wright TA, Kow L, Wilson T, Toouli J. Early results of laparoscopic swedish adjustable gastric banding for morbid obesity. Br J Surg. 2000;87:362-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Suter M, Giusti V, Worreth M, Héraief E, Calmes JM. Laparoscopic gastric banding: a prospective, randomized study comparing the Lapband and the SAGB: early results. Ann Surg. 2005;241:55-62. [PubMed] |
| 81. | Ponson AE, Janssen IM, Klinkenbijl JH. Laparoscopic adjustable gastric banding: a prospective comparison of two commonly used bands. Obes Surg. 2002;12:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Fried M, Miller K, Kormanova K. Literature review of comparative studies of complications with Swedish band and Lap-Band. Obes Surg. 2004;14:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Yang Y, Kuwano H, Okudaira Y, Kholoussy AM, Matsumoto T. Use of intragastric balloons for weight reduction. An experimental study. Am J Surg. 1987;153:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Cigaina V, Pinato G, Rigo V, Bevilacqua M, Ferraro F, Ischia S, Saggioro A. Gastric Peristalsis Control by Mono Situ Electrical Stimulation: a Preliminary Study. Obes Surg. 1996;6:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12 Suppl 1:12S-16S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res. 2003;11:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Miller KA. Implantable electrical gastric stimulation to treat morbid obesity in the human: operative technique. Obes Surg. 2002;12 Suppl 1:17S-20S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Shikora SA. Implantable gastric stimulation for weight loss. J Gastrointest Surg. 2004;8:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345-1351. [PubMed] |
| 91. | Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1550] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 92. | Howard L, Malone M, Michalek A, Carter J, Alger S. Gastric Bypass and Vertical Banded Gastroplasty- a Prospective Randomized Comparison and 5-Year Follow-up. Obes Surg. 1995;5:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Linner JH. Comparative effectiveness of gastric bypass and gastroplasty: a clinical study. Arch Surg. 1982;117:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Brolin RE, Kenler HA, Gorman JH, Cody RP. Long-limb gastric bypass in the superobese. A prospective randomized study. Ann Surg. 1992;215:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 269] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 95. | Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass. Am J Surg. 1996;171:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339-50; discussion 350-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1469] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 97. | Fobi MA, Lee H, Holness R, Cabinda D. Gastric bypass operation for obesity. World J Surg. 1998;22:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 98. | Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic Gastric Bypass, Roux-en-Y: Preliminary Report of Five Cases. Obes Surg. 1994;4:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 465] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 99. | Higa KD, Boone KB, Ho T, Davies OG. Laparoscopic Roux-en-Y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg. 2000;135:1029-1033; discussion 1033-1034;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 100. | Schauer P, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. 2003;17:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 101. | Shikora SA, Kim JJ, Tarnoff ME, Raskin E, Shore R. Laparoscopic Roux-en-Y gastric bypass: results and learning curve of a high-volume academic program. Arch Surg. 2005;140:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, Wolfe BM. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279-289; discussion 289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 632] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 103. | Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 828] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 104. | Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients--what have we learned. Obes Surg. 2000;10:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 105. | Wittgrove AC, Clark GW. Laparoscopic gastric bypass: endostapler transoral or transabdominal anvil placement. Obes Surg. 2000;10:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 106. | Farkas DT, Vemulapalli P, Haider A, Lopes JM, Gibbs KE, Teixeira JA. Laparoscopic Roux-en-Y gastric bypass is safe and effective in patients with a BMI > or =60. Obes Surg. 2005;15:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Tichansky DS, DeMaria EJ, Fernandez AZ, Kellum JM, Wolfe LG, Meador JG, Sugerman HJ. Postoperative complications are not increased in super-super obese patients who undergo laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2005;19:939-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Helling TS. Operative experience and follow-up in a cohort of patients with a BMI > or =70 kg/m2. Obes Surg. 2005;15:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Eisenberg D, Bell RL The impact of bariatric surgery on severely obese patients with diabetes. Diabetes Spectrum. 2003;16:240-245. [DOI] [Full Text] |
| 111. | Foley EF, Benotti PN, Borlase BC, Hollingshead J, Blackburn GL. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg. 1992;163:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 397] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 113. | Sjöström CD, Peltonen M, Wedel H, Sjöström L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 213] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
S- Editor Pan BR E- Editor Bai SH