Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.287
Revised: June 28, 2005
Accepted: July 1, 2005
Published online: January 14, 2006
AIM: To study the role of N-acetylcysteine (NAC) as a protective agent in rifampicin (RMP)-induced oxidative hepatic injury of young rats.
METHODS: Hepatic injury was produced by giving 50mg/kg body weight/day of RMP for 3 wk. A dose of NAC (100mg/kg body weight/day) was given in combination with RMP intraperitoneally. Analysis of lipid peroxidation, thiol levels, cytochrome P450, superoxide dismutase (SOD), catalase, glutathione peroxidase, reductase and transferase were estimated in liver along with the body weight, liver weight and histological observations.
RESULTS: RMP exposure resulted in no change in body and liver weight while antioxidative enzymes were altered but the non protein thiol (GSH) status was well preserved. Cytochrome P450 system and peroxidation of lipids were induced by RMP exposure. Partial protection was observed with NAC against RMP-induced changes in liver, which was evidenced from the prevention of increase in lipid peroxidation and the reduction in SOD and catalase enzyme levels.
CONCLUSION: NAC protects young rats against RMP-induced oxidative hepatic injury.
- Citation: Rana S, Attri S, Vaiphei K, Pal R, Attri A, Singh K. Role of N-acetylcysteine in rifampicin-induced hepatic injury of young rats. World J Gastroenterol 2006; 12(2): 287-291
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.287
Tuberculosis is one of the major health problems in developing countries like India. Most adult deaths in India are due to vascular diseases or pulmonary tuberculosis[1]. Steel et al[2] reported that clinical hepatitis occurs in 1.1% of adults receiving RMP containing regimens. Occasional cases of RMP-associated hepatitis have been reported in patients not receiving isoniazid (INH) treatment[3]. The actual incidence of RMP-induced hepatitis remains unclear because RMP is always used in combination with other antitubercular drugs.
The mechanism of RMP-induced liver injury is not yet fully understood. Gangadharan[4] showed that RMP causes a direct toxic injury to the hepatocytes. This has been confirmed in experimental rats by Sodhi et al[5]. Hepatotoxicity still remains a definite yet unpredictable risk during treatment of tuberculosis patients, suggesting that diminution of protective mechanisms and enhancement of destructive mechanism might be important in RMP-induced hepatotoxicity.
It is well established that by augmenting cellular antioxidative defense system especially non protein thiols, i.e. glutathione (GSH), cells can be protected against oxidative injuries produced by various drugs[6]. Among the possible antioxidant chemicals, NAC known to be non-toxic has been used in the treatment of various disorders [7,8]. The present study was designed to see whether NAC could protect against RMP-induced oxidative hepatic injury in an experimental model.
Young male Wistar rats weighing about 150 - 200 g were selected in the present study. Animals were fed with standard rat pellet diet (Aashirwad Industries, Chandigarh) and water ad libitum.
NAC was obtained from Sigma Chemical Company, USA. RMP and NAC were administered intraperitoneally (i.p) to animals. RMP solution was prepared by dissolving the powder in sterile distilled water and then the pH was adjusted to 3.0 with 0.1 N HCI to have a clear solution[9]. Similarly, NAC was dissolved in sterile distilled water. The volume of sterile water injected either alone or containing drugs, was kept constant (4.0mL/kg body weight/day) in all the treatments.
A dose of NAC (100 mg/kg body weight/day) was selected when the animals treated with this dose did not exhibit any histopathological liver injury at the end of the treatment schedule.
Forty animals were divided into four groups (10 each group) and injected with RMP and / or NAC (dissolved in sterile water) for a period of three weeks.
Animals in control group were injected with sterile water alone. Animals in RMP group were injected with 50mg/kg body weight/day of RMP alone after dissolved in sterile distilled water. Animals in RMP+NAC group were injected with RMP (50mg/kg body weight/day) and NAC (100mg/kg body weight/day). Animals in NAC group were treated with NAC at a dose of 100mg / kg body weight / day.
Animals were fasted for 12 h before sacrificed by cervical dislocation under light anesthesia and weighed on d 0 and 10 and at the time of sacrifice. Their liver tissues were excised and cooled in an ice-bath after weighed. Liver tissues were perfused with ice-cold saline. Homogenate and post mitochondrial supernatant were prepared as previously described[5]. Crude homogenate (20%) was prepared in 100mmol/L phosphate buffer and used to estimate thiols (total and protein-bound) and lipid peroxidation. The homogenate was then centrifuged at 10000r/min for 20 min at 4°C. The post mitochondrial supernatant (PMS) was separated from the pellet by decantation and pellet was discarded. All enzyme assays were carried out using PMS fraction.
Estimation of thiols was carried out according to the method of Sedlak and Lindsay[10]. Lipid peroxidation was measured by the method of Ohkawa et al[11]. Cytochrome P450 was assayed using the method of Omura and Sato [12]. The method of Kono[13] was adopted to estimate superoxide dismutase. Activity of catalase was measured by the method of Luck[14]. Glutathione peroxidase, glutathione reductase and glutathione transferase were estimated using the methods of Flohe and Gungler[15], Carlberg et al [16] and Habig et al [17], respectively. Proteins were estimated using bovine serum albumin as standard by the method of Lowry et al [18].
Small pieces of liver tissue from sacrificed animals were preserved in 10% formal saline. Light microscopy was performed after slides were routinely stained with haematoxylin and eosin (H&E).
Data were analysed by analysis of variance (ANOVA) followed by multiple comparison using Dunnett’s procedure to compare all groups against control and Student-Newman-Keul’s procedure to compare the pair wise of all groups.
An approval from Postgraduate Institute of Medical Education and Research Ethical Committee was obtained before the animal experimentation.
Analysis of variance showed that inter-group variation in body weight and the effect of treatment on body weight were not significant. Relative liver weight (g/100 g body weight) of experimental animals was not affected by any of the treatments. Animals treated with RMP had comparable liver weight (4.75 ± 0.75g) to animals treated with RMP+NAC (4.85 ± 0.67g).
Mortality in RMP treatment group was decreased from 30% (3/10) to 10% (1/10) when NAC was co-administered with RMP.
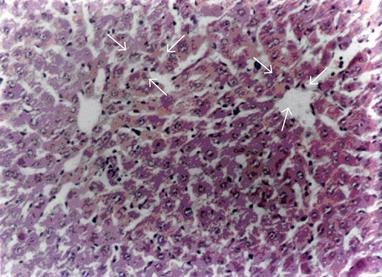
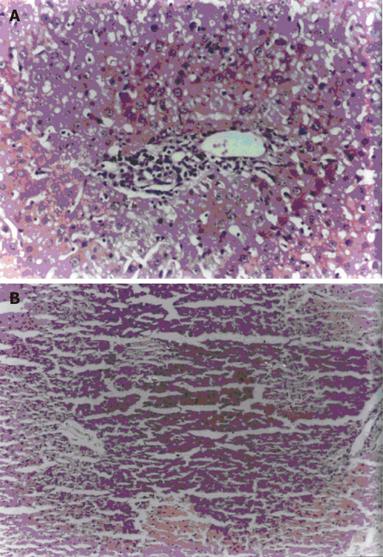
Essentially normal morphology was observed in all 10 control animals (100%) (Figure 1). Hepatotoxicity was produced by i.p. injection of RMP at a dose of 50mg / kg body weight / day over a period of three weeks as evidenced by the presence of changes such as hepatocytic necrosis and portal triaditis. Nine out of 10 (90%) animals treated with RMP showed histological lesions, 7 out of 10 (70%) animals had moderate degree of portal triaditis (Figure 2A) and 2 out of 10 (20%) had spotty necrosis with portal triaditis (Figure 2B). Only 1 out of 10 (10%) animals treated with RMP showed normal morphology.
Animals co-exposed to RMP+NAC exhibited lower degrees of histological lesions such as mild to moderate portal triaditis and spotty necrosis. Control animals treated with NAC alone had no change in liver histology.
Hepatic thiols were significantly increased (P<0.05) after treatment with RMP, while NAC had no effect on total and bound thiols when animals were treated with RMP+NAC. Multiple comparisons using Newman-Keul’s procedure did not reveal any significant change in treated groups with respect to non protein thiols (GSH) (Table 1).
| Group | Total thiols | Protein bound | Non protein thiols(GSH) |
| Control | 13.42 ± 0.52 | 10.09 ± 0.45 | 3.23 ± 0.42 |
| RMP | 16.77 ± 0.61a | 13.49 ± 0.42a | 2.98 ± 0.61 |
| RMP + NAC | 17.17 ± 0.78b | 13.92 ± 0.51b | 3.37 ± 0.72 |
| NAC | 13.44 ± 0.50 | 9.55 ± 0.25 | 3.42 ± 0.43 |
Lipid peroxidation was significantly (P<0.001) enhanced in animals exposed to RMP compared to the controls. Simultaneous administration of NAC and RMP prevented the enhancement of lipid peroxidation compared to control animals (P<0.05). But the values of lipid peroxidation in RMP+NAC-treated animals were higher (P<0.05) than those in the controls (Table 2). There was no significant difference in LPO values between NAC-treated group and controls.
| Group | Lipid peroxidation (nmolMDA/g tissue/10min) | Catalase(µmol/min/mgprotein) | Superoxidedismutase (IU) | Glutathione-S-transferaseusing EA |
| Control | 120.47±9.3 | 310.32±31.2 | 17.92±2.25 | 13.94±2.27 |
| RMP | 163.52±22.6b | 204.92±28.6b | 11.71±1.39b | 18.58±1.52Wb |
| RMP + NAC | 138.71±13.2Wa | 269.48±19.5Wa | 14.38±2.47Wa | 15.92±1.38a |
| NAC | 103.59±12.9 | 317.51±38.7a | 16.47±2.80 | 14.59±2.18 |
Superoxide dismutase (SOD) was decreased significantly (P<0.001) in RMP-treated animals compared to controls. Supplementation of NAC to RMP treated animals significantly prevented the reduction of superoxide dismutase while no effect of NAC was observed in control group (Table 2).
Catalase activity was significantly decreased in RMP-treated animals as compared to controls (P<0.001). Supplementation of NAC to RMP-treated animals prevented the lowering of catalase levels. However, animals treated with NAC in combination with RMP had significantly lower (P < 0.001) enzyme levels compared to the controls while NAC alone had no effect on catalase activity in control group (Table 2).
Levels of glutathione-S-transferase were significantly higher (P < 0.001) in RMP-treated group than in controls. Supplementation of NAC to RMP-treated animals prevented the increase of GST activity. GST level in NAC-treated animals was comparable to that in the controls (Table 2).
Glutathione peroxidase activity was significantly (P < 0.001) decreased after RMP treatment. NAC incombination with RMP could prevent the decrease of glutathione peroxidase activity but was still significantly lower than that in the controls (P < 0.05). NAC had no effect on this enzyme in control group (Table 3).
| Group | Glutathioneperoxidase | Glutathionereductase | Cytochrome P450 |
| Control (C) | 458.37±45.19 | 25.37±3.82 | 0.431±0.050 |
| RMP | 287.18±39.82Wb | 27.01±2.75 | 0.540±0.039b |
| RMP + NAC | 385.99±50.47a | 27.47±3.27 | 0.509±0.042a |
| NAC | 474.07±37.92 | 26.93±4.01 | 0.412±0.047 |
Glutathione reductase did not significantly increase in the RMP-treated animals compared to the controls. Supplementation of NAC to RMP-treated and control groups had comparable enzyme activities to non RMP-exposed animals (Table 3).
Cytochrome P450 was induced in RMP-treated animals (P < 0.001). Supplementation of NAC to RMP-treated and control animals had no effect on cytochrome P450 in the present study. The values in RMP+NAC group were comparable to those in RMP-treated animals (Table 3).
Rat model was used to study the hepatotoxic effect of RMP and hepatoprotective effect of NAC. Rats have been successfully used to establish RMP-induced hepatotoxicity models[9,19]. Our earlier studies have shown that oxidative stress is the mechanism of RMP-induced hepatotoxicity in experimental rats[5].
In the present study, hepatotoxicity was produced by RMP at a dose of 50 mg/kg body weight/day for three weeks. The dose is very high compared to that in the treatment of tuberculosis in human subjects, because higher doses are required for animals like rats as they metabolize the drugs at a faster rate[20].
In the present study, the drugs used had no effect on body weight and relative liver weight of the animals. This is consistent with what has been reported earlier[21].
Cytochrome P450 mediates generation of reactive metabolites of drugs and their covalent binding to hepatic macromolecules is the most accepted mechanism of RMP-induced hepatic injury[22]. RMP is a potent inducer of cytochrome P450 and enhances the covalent binding of reactive metabolites of acetyl hydrazine to the macromolecules of hepatocytes[21].
In the present study, free radicals formed either by the reaction of drug’s radicals with oxygen or by the interaction of superoxide radicals with hydrogen peroxide seemed to initiate lipid peroxidation in RMP-treated rats, suggesting that increased lipid peroxidation might be associated with cellular damage. NAC combined with RMP prevented significantly the peroxidation of lipids in animals exposed to RMP either directly or through non-protein thiols (GSH) by scavenging the radicals. The scavenging ability of NAC has also been reported during cancer chemotherapy [23,24]. From these studies and results of the present study, it can be concluded that the protective effect of NAC may be due to the radicals scavenged by NAC.
SOD, catalase and glutathione peroxidase constitute a mutually supportive team of antioxidative enzymes which provide a defense against reactive oxygen species. In the present study, SOD decreased significantly in RMP-exposed animals. The activities of H2O2 scavengers, catalase and glutathione peroxidase, decreased significantly after RMP treatment. Similar results have been reported by Sodhi et al [5]. The decline in these enzymes in the present study could be explained by the fact that excess superoxide radicals may inactivate H2O2 scavengers, thus resulting in inactivation of SOD[25].
In the present study, co-administration of NAC and RMP partially prevented decrease in catalase and glutathione peroxidase activities, which might be due to incomplete scavenging of radicals by NAC, resulting in partial protection of these enzymes. As NAC reacts with scavenging radicals slowly, residual superoxide radicals might interact with H2O2 resulting in the formation of OH radicals. This is supported by the fact that lower increase in lipid peroxidation was observed in NAC+RMP co-exposed animals as compared to RMP-treated animals. Jaya et al [26] have also reported that anti-peroxidative enzymes can be partially restored with NAC in rats exposed to alcohol and paracetamol.
It was reported that thiols increase significantly after RMP treatment, which might be due to the increased protein content[28].
GSH constitutes approximately 90% of total hepatic thiols, which could explain the maintained GSH levels in RMP-exposed animals in the present study. Supplementation of NAC also failed to increase the threshold levels of GSH. These results are inconsistent with Yao et al [27] who failed to demonstrate a significant rise of GSH in livers of rats treated with NAC.
In the present study, glutathione used in scavenging hydroxyl radicals stimulated glutathione reductase activity, thereby maintaining nearly normal GSH. NAC failed to decrease glutathione reductase activity in RMP-exposed animals. These results might be due to stimulation of glutathione reductase activity by the low level of glutathione and or a negative feedback inhibition by the normalization of GSH levels in NAC treated animals.
GST activity against EA was stimulated in animals treated with RMP and RMP+NAC. GST is specific for removal of peroxidation products and induction of this enzyme might be in response to lipid peroxidation so as to remove the highly toxic materials from cellular milieu. Adachi et al [28] reported that RMP can induce GST activity in rats.
The histopathological patterns of liver injury in the present study are similar to the earlier findings[9,29]. Sodhi et al [5] also showed that patchy necrosis occurs in RMP-treated animals. In the present study, NAC failed to protect completely against RMP-induced hepatic injuries but it protects completely against hepatic injuries in rats after treatment with INH+RMP[30].
In conclusion, RMP exposure to animals does alter the profile of antioxidant enzymes while non-protein thiol status can be well preserved. The protective effect of NAC on RMP-induced hepatic injury might be due to prevention of lipid peroxidation as well as decline in superoxide dismutase in animals exposed to RMP and NAC. However, partial hepatotoxicity due to RMP might be due to some other mechanisms of injury.
Lupin Laboratories, India, was acknowledged for supplying the purified RMP powder as a gift.
| 1. | Gajalakshmi V, Peto R, Kanaka TS, Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 287] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Bachs L, Parés A, Elena M, Piera C, Rodés J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102:2077-2080. [PubMed] |
| 4. | Gangadharan PRJ. Isoniazid, rifampicin and hepatotoxicity. Ann Rev Respir Dis. 1986;133:963-965. |
| 5. | Sodhi CP, Rana SF, Attri S, Mehta S, Yaiphei K, Mehta SK. Oxidative-hepatic injury of isoniazid-rifampicin in young rats subjected to protein and energy malnutrition. Drug Chem Toxicol. 1998;21:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Farrell GC. Biochemical mechanisms. In : Khullar S, Hunter S drug induced eds. Liver disease. New York: Churchill Livingstone 1994; 247-255. |
| 7. | Meyer A, Buhl R, Kampf S, Magnussen H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am J Respir Crit Care Med. 1995;152:1055-1060. [PubMed] |
| 8. | Nakano H, Boudjema K, Jaeck D, Alexandre E, Imbs P, Chenard MP, Nagasaki H, Kumada K, Wolf P, Cinqualbre J. Amelioration of hepatocellular integrity and inhibition of sinusoidal oxidative stress by N-acetylcysteine pretreatment in cold ischemia-reperfusion injury of rat liver. Eur Surg Res. 1996;28:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Bahri AK, Chiang CS, Timbrell JA. Acetylhydrazine hepatotoxicity. Toxicol Appl Pharmacol. 1981;60:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5089] [Cited by in RCA: 5512] [Article Influence: 95.0] [Reference Citation Analysis (6)] |
| 11. | Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem Biophys Res Commun. 1994;201:508-515. [PubMed] |
| 12. | Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370-2378. [PubMed] |
| 13. | Konno Y. Generation of Superoxide radical during auto-oxidation of dyhydroxylamine and an assay for Superoxide dismutase. Arch Biochem Biophys. 1978;186:189-195. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1228] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 14. | Luck H. Catalase estimation. New York & London: Academic Press 1971; 886-889. |
| 15. | Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3210] [Cited by in RCA: 3456] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 16. | Carlberg I, Mannervik B. Purification by affinity chromatography of yeast glutathione reductase, the enzyme responsible for the NADPH-dependent reduction of the mixed disulfide of coenzyme A and glutathione. Biochim Biophys Acta. 1977;484:268-274. [PubMed] |
| 17. | Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139. [PubMed] |
| 18. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 19. | Zitkowa L, Stastna J, Dobrousky K, Tousak J. Causes for rifampicin hepatoxicity: An experimental study. Czechosolvak Med. 1982;5:210-217. |
| 20. | Pelikan EW. Cited by Bushly SRM. Toxicity in chemotherapy. Experimental chemotherapy. New York: Academic Press 1963; 25-52. |
| 21. | Powell-Jackson PR, Tredger JM, Smith HM, Davis M, Williams R. Effect of isoniazid administration on selected rat and mouse hepatic microsomal mixed-function oxidases and in vitro [14C]acetylhydrazine-derived covalent binding. Biochem Pharmacol. 1982;31:4031-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Sinha BK. Activation of hydrazine derivatives to free radicals in the perfused rat liver: a spin-trapping study. Biochim Biophys Acta. 1987;924:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Umemura T, Hasegawa R, Sai-Kato K, Nishikawa A, Furukawa F, Toyokuni S, Uchida K, Inoue T, Kurokawa Y. Prevention by 2-mercaptoethane sulfonate and N-acetylcysteine of renal oxidative damage in rats treated with ferric nitrilotriacetate. Jpn J Cancer Res. 1996;87:882-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ercal N, Treeraphan P, Hammond TC, Matthews RH, Graneman NH, Spitz . H. In vivo indexes of oxidative stress in lead exposed C57 BL/6 mice are reduced by treatment with meso-9, 3-dimercaptosuccinic acid or N-acetylcysteine. Free Radicals Biol Med. 1996;21:157-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Blech DM, Borders CL. Hydroperoxide anion, HO-2, is an affinity reagent for the inactivation of yeast Cu,Zn superoxide dismutase: modification of one histidine per subunit. Arch Biochem Biophys. 1983;224:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Jaya DS, Augustine J, Menon VP. Protective role of N-acetylcysteine against alcohol and paracetamol induced toxicity. Ind J Clin Biochem. 1994;9:64-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Yao WB, Zhao YQ, Abe T, Ohta J, Ubuka T. Effect of N-acetylcysteine administration on cystein and glutathione contents in liver and kidney and in perfused liver of intact and diethylmaleate treated rats. Amino Acids. 1994;7:255-266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Adachi Y, Nanno T, Yamashita M, Ueshima S, Yamamoto T. Induction of rat liver bilirubin-conjugating enzymes and glutathione S-transferase by rifampicin. Gastroenterol Jpn. 1985;20:104-110. [PubMed] |
| 29. | Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA, Snodgrass WR, Nelson SD. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976;84:181-192. [PubMed] |
| 30. | Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, Nain CK, Singh K. Isoniazid- and rifampicin-induced oxidative hepatic injury--protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517-522. [PubMed] |
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Wu M