Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.271
Revised: July 28, 2005
Accepted: July 28, 2005
Published online: January 14, 2006
AIM: To characterize the consequences of short-term exposure to luminal bile on mucosal mast cell reactions in a canine model, and to determine the effects of systemic phosphatidylcholine pretreatment in this condition.
METHODS: Twenty mongrel dogs were used for experiments. Group 1 (n = 5) served as a saline-treated control, while in group 2 (n = 5) the esophagus was exposed to bile for 3 h. In group 3 (n = 5) the animals were pretreated with 7-nitroindazole to inhibit the neuronal isoform of nitric oxide synthase. In group 4 (n = 5) phosphatidylcholine solution (50 mg/kg) was administered iv before the biliary challenge. Mucosal microcirculation was observed by intravital videomicroscopy. Myeloperoxidase and nitric oxide synthase activities, the degrees of mast cell degranulation and mucosal damage were evaluated via tissue biopsies.
RESULTS: Exposure to bile evoked significant mast cell degranulation and leukocyte accumulation. The red blood cell velocity and the diameter of the postcapillary venules increased significantly. The tissue ATP content and constitutive nitric oxide synthase activity decreased, while the inducible nitric oxide synthase activity increased significantly as compared to the control values. 7-nitroindazole treatment significantly exacerbated the mucosal mast cell degranulation and tissue damage. In contrast, phosphatidylcholine pretreatment prevented the bile-induced ATP depletion, the inducible nitric oxide synthase and myeloperoxidase activity and the mast cell degranulation increased.
CONCLUSION: The neuronal nitric oxide synthase - mast cell axis plays an important role in the esophageal mucosal defense system. Systemic phosphatidylcholine pretreatment affords effective protection through ameliorating the bile-induced ATP depletion and secondary inflammatory reaction.
- Citation: Eros G, Kaszaki J, Czobel M, Boros M. Systemic phosphatidylcholine pretreatment protects canine esophageal mucosa during acute experimental biliary reflux. World J Gastroenterol 2006; 12(2): 271-279
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.271
During acute regurgitation or prolonged gastroesophageal reflux episodes, the esophageal epithelial layer is exposed to various noxious luminal agents. Gastric acid has been shown to play a crucial role in the development of esophagitis, but regurgitated bile could also be linked to various detrimental mucosal reactions, including ATP depletion and permeability alterations [1,2]. Bile salts can damage the epithelium both directly and indirectly, and may alter the function of cells. Given that mast cells (MCs) are involved in stress-induced gastrointestinal reactions, it seems reasonable to assume that the effects of bile involve MCs. Indeed, it has been shown in vitro that bile acids induce concentration-dependent MC degranulation in correlation with their lipophilicity and surface activity [3].
A number of MC-specific reactions in the gastrointes-tinal tract may be linked to the activity of constitutively expressed nitric oxide synthase (NOS). A relative lack of NO can activate both MCs and leukocytes, and MC degranulation per se can bring about leukocyte accumul-ation and other characteristics of local inflammation [4,5] . NOS system consists of three distinct isoforms: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). Both eNOS and nNOS are constitutively expressed, but nNOS is predominant in the gastrointestinal tract [6,7]. Although the above line of reasoning suggests that MCs and nNOS-derived NO could be closely associated with mucosal homeostasis, the relative contributions of the different NOS isoforms to the reflux-caused inflammatory responses of the esophagus are unclear. In the first part of our study, therefore, we examined the consequences of an acute biliary challenge on NOS activation and mucosal MC reactions by performing detailed microcirculatory, histological and biochemical analyses. As we hypothesized that bile-induced inflammatory response critically involves NO, we determined the consequences of nNOS inhibition in this setting.
In addition to this pathomechanism investigation, another aim was to outline a means of modulating the outcome of biliary mucosal irritation. It is clearly recognized that a mixed or biliary reflux is more harmful than gastric acid alone, but to date there are no effective pharmacological therapies for bile-induced esophagitis. Nevertheless, various lines of indirect evidence have suggested that phosphatidylcholine (PC) may be protective during this condition. It was postulated in early in vitro studies that PC in aqueous media may protect against the membrane damage caused by bile salts [8,9]. In stress conditions the hydrolysis of endogenous membrane PC leads to release of phosphatidic acid and choline. Choline is anti-inflammatory and is actively transported into the epithelial cells [10]. Moreover, choline could form part of a defense mechanism which may operate in biological systems against oxido-reductive stress [11].
Accordingly, in the second part of our study we set out to establish whether systemic PC administration can protect the esophageal mucosa by acting as an anti-inflammatory agent in bile-induced esophagitis.
The experiments were performed in adherence to the NIH guidelines for the use of experimental animals. The study was approved by the Ethical Committee for the Protection of Animals in Scientific Research at the University of Szeged.
The experiments were performed on 20 inbred mongrel dogs (average weight 12 ± 3 kg) under sodium pentobarbital anesthesia (30 mg/kg iv). Small supplementary doses of pentobarbital were administered when necessary. The left femoral artery and vein were cannulated for recording the mean arterial pressure and blood sampling, respectively. The animals were placed in a supine position on a heating pad for maintenance of the body temperature between 36°C and 37°C, and received an infusion of Ringer’s lactate at a rate of 10 mL/(kg h) during the experiments.
Following a collar incision, the cervical esophagus with intact neurovascular connections was dissected free, and an approximately 8-10-cm segment of the middle portion was then occluded at both ends with atraumatic clips. The objective of the videomicroscope (Cytoscan A/R, Cytometrics, PA., USA) was placed into the esophagus lumen for continuous observation of the microcirculation. A second polyvinyl tube (0.5 mm i. d.) was secured with purse-string sutures for administration of the test compounds.
Surgery was followed by a 30-min recovery period for cardiovascular stabilization, and 7.0 mL isotonic saline (pH 7.4) was then injected into the lumen of the dissected esophagus for 30 min in order to determine the baseline variables. The esophageal segment was next filled with test solution (7.0 mL) for 180 min. At the end of the experiment, a biopsy was taken from the esophageal segment, together with a tissue sample from the aboral intact part of the esophagus, with a freeze-clamp technique for determination of the tissue adenosine triphosphate (ATP) concentrations, and additional biopsies were performed to measure the tissue myeloperoxidase (MPO) and NOS isoform activities, and to determine the extent of MC degranulation and structural damage.
The animals were randomly allotted into 4 groups. Group 1 (n = 5) served as saline-treated control, while in groups 2-4 the effects of bile were investigated. In group 2 (n = 5) the animals were treated with canine bile alone. Bile obtained from 3 healthy dogs was pooled and stored at -20oC, and diluted freshly (pH 6.5) before the experiments. Bile was diluted with same volume of saline and solution was warmed up to 37oC before administration. Group 3 (n = 5) was treated with 5 mg/kg 7-nitroindazole (7-NI, Sigma Chem., USA) in 0.3mL/min iv infusion 20 min before bile administration. In group 4 (n = 5) the animals received 50 mg/kg iv PC infusion 20 min before bile treatment. Five percent PC solution (soybean lecithin, MW: 785, phospholipon 90, Phospholipid GmbH, Cologne, Germany) was freshly prepared according to the description of the manufacturer. Briefly, PC solution contained deoxycholate (2.3%), NaOH (0.24%), benzyl alcohol (0.82%), NaCl (0.22%), and ethanol (0.27%) in distilled water. The intraluminal volume load was identical in all groups studied.
Central venous pressure and mean arterial pressure were measured continuously with Statham P23Db transducers and registered with a computerized data-acquisition system (Haemosys 1.17, Experimetria Ltd., Budapest, Hungary). Arterial blood gases were measured with a blood gas analyzer (AVL Compact 2, Graz, Austria).
The intravital OPS technique (Cytoscan A/R, Cytometrics, PA, USA) was used for continuous visualization of the microcirculation of the esophageal mucosa. This technique utilizes reflected polarized light at the wavelength of the isosbestic point of oxy- and deoxyhemoglobin (548 nm). Since polarization is preserved in reflection, only photons scattered from a depth of 2-300 μm contribute to the image formation. A 10x objective was introduced into the intestinal lumen, and the microscopic images were recorded with a S-VHS video recorder (Panasonic AG-TL 700). Videomicroscopic observations were made at specific anatomic locations at a depth of approximately 200 µm. Microcirculatory evaluation was performed off-line by frame-to-frame analysis of the videotaped images. Capillary red blood cell velocity (RBCV, µm/s), vessel diameter (VD, µm) changes in postcapillary venules and relative vessel area (RVA, length of perfused nutritive capillaries per total capillary length of a standard observation area) were determined in 3 separate fields by means of a computer-assisted image analysis system (IVM Pictron, Budapest, Hungary). Changes in venular wall diameter were determined by following identifiable visual landmarks within the vessel wall. All microcirculatory evaluations were performed by one investigator (EG).
A whole-thickness sample was taken from the esophagus with a Wollenberg forceps cooled in liquid nitrogen, and the tissue was stored at -70 oC. The sample was weighed, placed into a 3-fold volume of trichloroacetic acid (6% w/v), homogenized for 1 min, and centrifuged at 5000 g/min. The supernatant was neutralized with saturated potassium carbonate solution. The ATP concentration was measured spectrophotometrically according to Lamprecht et al [12]. The method is based on the principle that β-nicotinamide adenine dinucleotide phosphate is used up in an enzymatic reaction catalyzed by glucose-6-phosphate dehydrogenase and hexokinase.
NO formation in esophageal tissue was measured by the conversion of [3H] L-citrulline from [3H] L-arginine according to the method of Szabo et al[13]. Briefly, tissue homogenates were centrifuged at 24 000 g/min for 20 min at 4 °C and the supernatant was loaded into centrifugal concentrator tubes (Amicon Centricon-100; 100 000 MW cut-off ultrafilter). The tubes were centrifuged at 1000 g/min for 150 min and the concentrated supernatant was washed out from the ultrafilter with 300 μL homogenizing buffer. The samples were incubated with a cation-exchange resin (Dowex AG 50W-X8, Na+ form) for 5 min to deplete endogenous L-arginine. The resin was separated by centrifugation at 1500 g/min for 10 min and the supernatant containing the enzyme was assayed for NOS activity. For the Ca2+-dependent NOS (cNOS) activity, 50 μL enzyme extract and 100 μL reaction mixture (pH 7.4, containing 50mmol/L Tris-HCl buffer, 1mmol/L NADPH, 10 μmol/Ltetrahydrobiopterin, 1.5 mmol/L CaCl2, 100 U/mL calmodulin and 0.5 μCi [3H] L-arginine (ICN Biomedicals, specific activity 39 Ci/mmol) were incubated together for 30 min at 37 oC. The reaction was stopped by the addition of 1 mL ice-cold HEPES buffer (pH 5.5) containing 2mmol/L EGTA and 2mmol/L EDTA. Measurements were performed with boiled enzyme and the NOS inhibitor N-ω-nitro-L-arginine (3.2mmol/L) to determine the extent of [3H] L-citrulline formation that was independent of the NOS activity. Ca2+-independent NOS activity (iNOS) was measured without Ca-calmodulin but with EGTA (8 mmol/L). Reaction mixture (1 mL) was applied to Dowex cation-exchange resin (AG 50W-X8, Na+ form) and eluted with 2 mL distilled water. The eluted [3H] L-citrulline activity was measured with a scintillation counter (Tri-Carb Liquid Scintillation Analyze 2100TR/2300TR, Packard Instrument Co, Meriden, Ct., USA) and referred to the protein content.
The activity of MPO, a marker of tissue leukocyte infiltration, was measured from mucosal biopsies by the method of Kuebler et al [14]. Briefly, the tissue was homogenized with Tris-HCl buffer (0.1 mmol/L, pH 7.4) containing 0.1 mM polymethylsulfonyl fluoride to block tissue proteases, and then centrifuged at 2000 g/min at for 20 min at 4°C. The MPO activities of the samples were measured at 450 nm (UV-1601 spectrophotometer, Shimadzu, Japan), and the data were referred to the protein content.
Esophageal biopsy samples were rapidly placed into ice-cold Carnoy’s fixative, embedded in paraffin, sectioned (6 μm) and stained with hematoxylin-eosin. Histological analysis was performed in coded sections by one investigator (MB). Mucosal injury was graded on the 0-100 esophageal mucosal damage score of Lanas et al [15] with the following criteria: epithelial changes (epithelial splitting, erosion and ulceration): maximal score 40; inflammation (intraepithelial leukocytes and cellular hyperplasia): maximal score 40; vascular lesions (edema, congestion and hemorrhage): maximal score 20.
Counting of polymorphonuclear leukocytes (PMN) was performed on coded sections by two independent investigators (TL, EG). Five nonoverlapping fields were observed in each section, and in each field an average of 4 consecutive measurements was used to calculate average PMN count with a semiquantitative scoring system. PMN counts were determined in the epithelium, submucosa, muscle layer and adventitia with the following criteria: grade 0, no PMNs in the given structure; grade 1, 1 - 10 PMNs; grade 2, 11 - 100 PMNs; grade 3: > 100 PMNs.
The sections were stained with acidic toluidine blue (pH 0.5) and alcian blue-safranine O (Sigma Chem. USA, pH 0.4). Positively stained MCs were quantitated in 10 fields. The counting was performed in coded sections at an optical magnification of x 400 by two independent investigators (EG and TL). Loss of intracellular granules and stained material dispersed diffusely within the lamina propria were taken as evidence of MC degranulation. For each animal, 5 fields were chosen at random, and the number of degranulated and intact MCs was counted. All counts were pooled for each treatment, and the percentage of degranulated MCs was calculated.
Data analysis was performed with a statistical software package (SigmaStat for Windows, Jandel Scientific, Erkrath, Germany). Nonparametric methods were used. Friedman repeated measure analysis of variance on ranks was applied within the groups. Time-dependent differences from the baseline were assessed by Dunn’s method. Differences between groups were analyzed with Kruskal-Wallis one-way analysis of variance on ranks, followed by Dunn’s method for pairwise multiple comparison. In the Table and Figures, median values and 75th and 25th percentiles are given. P < 0.05 was considered statistically significant.
The baseline values of the macrohemodynamic variables did not differ significantly in the different groups and there were no significant hemodynamic changes as compared to the control values during the experimental period (Table 1). The mean arterial pressure in the bile+7-NI or PC-treated groups was not significantly different (P > 0.05) from that in the saline-treated group as a whole (Table 1).
| Group | Time | -30 min | 0 | 30 min | 60 min | 90 min | 120 min | 180 min |
| RBCV(M) | 600 | 608 | 572 | 615 | 569 | 575 | 630 | |
| 25p; 75p | 554; 661 | 548; 663 | 536; 662 | 526; 709 | 539; 673 | 524; 705 | 540; 705 | |
| VD (M) | 31 | 33 | 33 | 32 | 33 | 33 | 34 | |
| Saline | 25p; 75p | 27.3; 34.3 | 27.5;34.5 | 27.3;34.0 | 28.3; 34.8 | 28.3; 34.0 | 27.5; 34.0 | 31; 34 |
| MAP (M) | 163 | 161 | 158 | 157 | 150 | 156 | 152 | |
| 25p; 75p | 134.5;172 | 132; 175 | 130; 168 | 132;167 | 129;169 | 128; 168 | 129; 169 | |
| HR (M) | 172.5 | 171 | 169 | 167 | 177 | 180 | 181 | |
| 25p; 75p | 126; 200 | 145; 183 | 151; 206 | 141.5; 198 | 142;195 | 141; 194 | 146; 188 | |
| RBCV(M) | 621 | 662 | 717 | 783 a | 770 ac | 777 ac | 813 ac | |
| 25p; 75p | 573; 660 | 574; 693 | 610; 791 | 734; 821 | 686; 828 | 712; 836 | 728; 869 | |
| VD (M) | 33 | 33.5 | 41 ac | 45.5 ac | 43.0 ac | 42.5 ac | 43.5 ac | |
| Bile | 25p; 75p | 33.0; 34.0 | 33; 35.0 | 39; 43.0 | 41.0; 48.0 | 41.0; 45.0 | 42.0; 44.0 | 42.0; 47 |
| MAP (M) | 155 | 157 | 153 | 137 | 145 | 145 | 147 | |
| 25p; 75p | 129; 185 | 131; 181 | 128; 184 | 127;185 | 126; 176 | 121; 181 | 127; 185 | |
| HR (M) | 167 | 171 | 161 | 161 | 181 | 177 | 192 | |
| 25p; 75p | 160; 174 | 157; 191 | 149; 171 | 158; 170.5 | 141.5; 197 | 154; 197 | 153; 194 | |
| RBCV(M) | 600 | 651 | 789 c | 809 ac | 821 ac | 784 ac | 818 ac | |
| 25p; 75p | 554; 661 | 640; 703 | 698; 872 | 694; 888 | 715; 881 | 727; 806 | 724; 870 | |
| PC+ bile | VD (M) | 31 | 35 | 37.0 ce | 37.5 ce | 39.0 ce | 38.0 ce | 38.0 ce |
| 25p; 75p | 27.3; 34.3 | 32; 39.0 | 33.5; 40 | 35.5; 41.5 | 34.0; 41.0 | 33.0; 42.5 | 34.5; 42 | |
| MAP (M) | 163 | 147.5 | 161.5 | 152.5 | 150 | 162.5 | 160 | |
| 25p; 75p | 134.5;172 | 130; 154 | 133; 170 | 131; 167.5 | 129; 168.5 | 132; 174 | 133; 173 | |
| HR (M) | 172.5 | 162 | 164 | 162.5 | 171 | 180 | 188 | |
| 25p; 75p | 126; 200 | 145, 167 | 143; 180 | 148, 188 | 142; 199 | 158; 186 | 183; 198 | |
| RBCV(M) | 625 | 632 | 791 c | 762 a | 821 ac | 793 c | 872 ac | |
| 25p; 75p | 556; 696 | 614; 671 | 649; 870 | 661; 893 | 761; 870 | 718; 845 | 857; 893 | |
| 7-NI+ bile | VD (M) | 31 | 32 | 34.0 e | 34.0 e | 32.5 e | 34.0 e | 33.5 e |
| 25p; 75p | 30.0; 31.0 | 29; 33.0 | 33; 36 | 34.0; 34.0 | 31.0; 36.0 | 32.0; 34.0 | 32; 34.0 | |
| MAP (M) | 132.5 | 138 | 145 | 137.5 | 141.5 | 135.5 | 139.5 | |
| 25p; 75p | 128; 144 | 133; 150 | 132; 150 | 130; 151 | 134; 149 | 130; 143 | 135; 145 | |
| HR (M) | 170 | 163 | 171 | 165 | 181 | 185 | 186 | |
| 25p; 75p | 162; 180 | 159, 169 | 158; 182 | 152; 178 | 173; 191 | 176.5; 196 | 182; 189 |
Venules of 35 ± 10 µm in diameter were the largest fraction of the vessels and arterioles were seen only very rarely. The baseline level of RBCV in the various groups ranged 560 - 680 μm/s, and in the control group the RBCV did not change during the experiments (Table 1). However, the RBCV was increased significantly after 3-h exposure to bile with or without 7-NI or PC pretreatment. The median value was 813, 886 and 787 μm/s after bile, bile with 7-NI, and bile with PC pretreatment, respectively.
The inner boundary of the venular wall was easily distinguishable and an exact comparison of inner diameter changes was possible. The median vessel diameter was significantly greater in bile-treated group 2 as compared to the saline-treated control, and increased from the baseline value of 33 μm to 44 μm. Administration of 7-NI before bile treatment prevented this increase. PC pretreatment resulted in a significant vessel diameter elevation as compared to the control group, but the diameter changes in this group were significantly lower than those in the bile-treated group (Table 1).
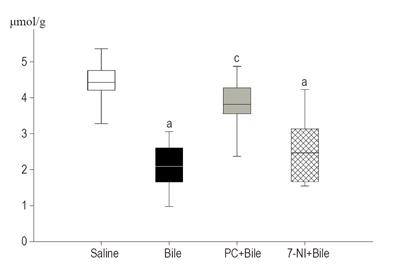
The in vivo interference of bile treatment with ATP production of the esophageal mucosa was evaluated (Figure 1). There were no significant differences in tissue ATP levels between the intact and treated parts of the esophagus in the saline-treated control group (data not shown). There was a statistically significant fall (45%) in the ATP content of the esophageal tissue after bile treatment (saline: M = 4.43 μmol/mg protein, 25p = 4.21, 75p = 4.76; bile: M = 2.42 μmol/mg protein, 25p = 1.66, 75p = 2.61) by the end of the observation period, and this change was not affected by 7-NI treatment. Following PC treatment, the ATP content was significantly maintained in the mucosa as compared to bile treatment alone (M = 3.82, 25p = 3.56, 75p = 4.28), and this value was not significantly different from the corresponding value in the saline-treated group.
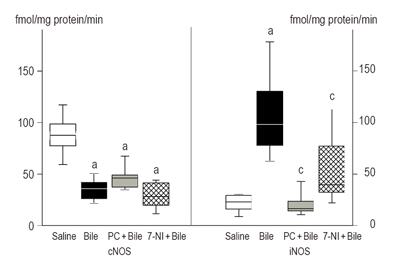
Figure 2 demonstrates the changes in esophageal cNOS and iNOS activities. The activity of cNOS was significantly depressed after bile treatment, and this change was accompanied with a significant increase (6-fold) in iNOS activity. The cNOS activity was not altered by PC or 7-NI pretreatment. However, the esophageal iNOS activity was significantly lower after PC pretreatment as compared to bile treatment alone. 7-NI treatment resulted in a somewhat lower increase (2.6-fold) in iNOS activity, as compared to the value in the bile-treated group.
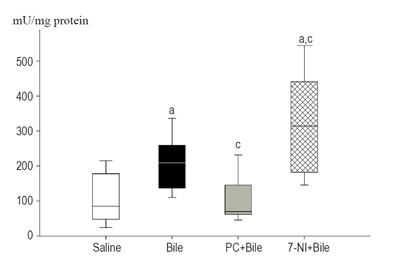
The MPO data demonstrated that leukocyte accumulation was significantly increased in mucosae of the bile-treated and 7-NI pretreated groups as compared to the saline-treated group (Figure 3). Bile alone resulted in a 2.5-fold (M = 209.4; 25p = 136.6; 75p = 259.6) rise in MPO activity, and a further increase was observed after 7-NI+bile administration (M = 315.4, 25p = 182.4, 75p = 441.8). PC pretreatment significantly decreased bile-induced MPO activity.
Histological scoring of leukocyte infiltration was performed by two independent viewers (the inter-observer variation was less than 15%, and these data showed good correlation with MPO results). In saline-treated group the extravasation of PMNs was negligible (range of scores: 1-3; M = 2). Exposure to bile resulted in a significant (P<0.05) accumulation of PMNs (range: 4-6; M = 5). In PC pretreated animals the PMN infiltration was significantly decreased and the scores did not differ significantly from the control values (range: 1-3; M = 3). Bile + 7-NI treatment increased the degree of PMN infiltration and resulted in a grade 7 score (range: 7-9).
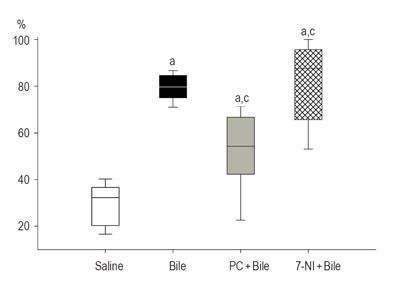
The effects of bile and various pretreatments on the mucosal MC degranulation are presented in Figure 4. In the control group, 35% of the MCs were degranulated. Exposure to bile resulted in a significant degranulation (M = 79.6%, 25p = 75.0, 75p = 84.6) relative to the control group. In the PC-pretreated group, the percentage of degranulated MCs (M = 54.3%, 25p = 42.2%, 75p = 66.7%) was significantly lower than that after bile treatment alone. The nNOS inhibition evoked a marked stimulation of MC degranulation over that due to bile treatment alone. In the 7-NI+bile-treated group, the degranulation of mucosal MCs was nearly complete (M = 87.5%, 25p = 65.6%, 75p = 95.6%).
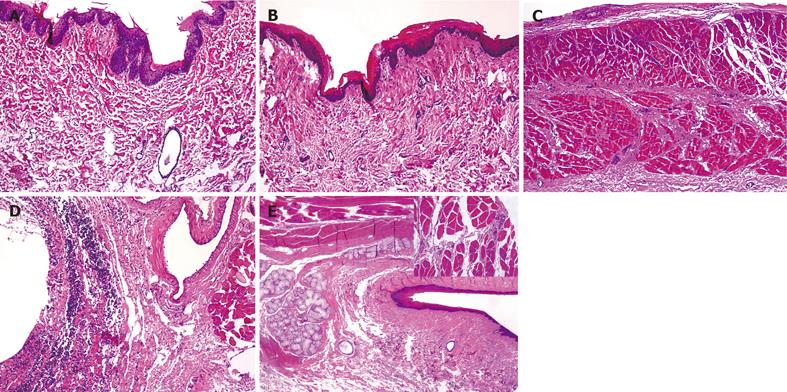
Biopsy samples from the saline-treated control group exhibited an average grade of injury of 5 (range of scores: 0 - 20) on the Lanas scale. In these sections, the luminal surface was always lined by a continuous layer of epithelial cells, while the vessels usually presented with empty lumina (Figure 5A). The 180-min bile exposure induced significant mucosal damage (P < 0.01), with a median value of 58 (range: 50 - 70). Deep lesions were observed with disruption and desquamation of the epithelial layer (Figures 5B and 5C). Extensive intraepithelial and subepithelial leukocytosis and connective tissue damage were the general characteristics. Submucosal edema, hemorrhage and vasodilatation were apparent. Semiquantitative evaluation of the samples from the 7-NI+bile-treated animals revealed a significant exacerbation of the mucosal injury (P < 0.01) and an injury score of 68 (range 60-90). A tendency toward more intense injury was always manifested. Severe epithelial damage and exfoliation of the surface epithelium were present, and the deeper tissue layers were more strongly involved in a generalized inflammatory reaction. In most cases, transmural inflammatory infiltrations, vasodilation and subepithelial connective tissue edema were observed (Figure 5D).
Administration of PC did not significantly influence the superficial tissue damage, but the reactive mucosal changes were different from those observed in the bile-treated group. In general, the degree of epithelial damage and the nature of reactive epithelial changes were similar to those observed in the bile-treated group 2, but there was no evidence of severe subepithelial inflammation. Submucosal damage was often patchy, mild and severe lesions were commonly observed within the same section and hemorrhage was rarely observed. The general image of tissue damage was somewhat improved, but this group had a median grade of injury of 50 (range: 40 - 75), a value not significantly different (P > 0.01) from the corresponding value for the bile-treated group.
Regurgitation of the duodenogastric content may lead to esophageal dysfunction and tissue damage in the long run, but acute biliary reflux likewise correlates with the presence of severe esophagitis and esophageal ulceration in critically ill and mechanically ventilated patients[16]. Appropriate treatment or prevention of this condition clearly presupposes an understanding of the pathogenesis of regurgitation-induced lesions and the nature of inflammatory complications.
The results of our in vivo study indicate that bile may target several potentially interconnected pathways, leading to mucosal barrier impairment. Intraluminal bile reduced ATP content of the exposed tissues, decreased cNOS activity, increased iNOS activity, evoked a parallel rise in MC degranulation and leukocyte accumulation, and induced severe structural alterations. These effects were potentiated by 7-NI, a selective inhibitor of the nNOS isoform.
NO plays a central role in the maintenance of resting esophageal mucosal blood flow, but it has been shown that the reactive responses to luminal deoxycholate are not NO-dependent [17,18]. In our experiments the acute biliary challenge elicited characteristic microcirculatory changes in the esophageal mucosa, as demonstrated by intravital videomicroscopy. Both the venular diameter and RBCV increased, which shows that a substantial venodilator influence is present in the mucosa, despite cNOS inhibition. This finding is seemingly controversial. Nevertheless, it is clear that other mechanisms or mediators could compensate for the absence of cNOS-derived NO. Nonspecific inhibitors of NOS generally increase the intestinal epithelial permeability, these changes are attributed to the destabilizing effects of NO deficit on MCs [4,19].
It is reasonable to suggest that MC-related histamine is a likely candidate in this process. Mucosal mast MCs are a unique cellular source of both preformed and de novo synthesized mediators, and MC-induced reactions contribute to postischemic mucosal permeability alterations and flow response in the gastrointestinal tract [5,20]. Moreover, it has been shown that bile acids are able to degranulate MCs and induce histamine release from mucosal MCs both in vitro and in vivo [3,21]. It has further been reported that MC-derived histamine is involved in esophageal and gastric vasodilatation during acid-induced injury [22]. This type of vasodilation could protect the mucosa against further injury and appears to be mediated by calcitonin gene-related peptides (CGRP) [23]. Thus, it is reasonable to suggest that bile-induced venodilatory response is closely associated with MC degranulation.
The current study also showed that bile potently inhibits the constitutive, Ca2+-dependent isoforms of NOS, even though the activity of the inducible isoform increased over time. The latter phenomenon could be a compensatory event of cNOS inhibition, or an aftermath of inflammatory stimuli, as iNOS has been shown to be present in macrophages and MCs too. The functioning of inducible, Ca2+-independent iNOS is closely related to the activation of NF-κB, and it has recently been shown that the iNOS mRNA expression increases within 30 min after an inflammatory challenge. Moreover, even physiological levels of deoxycholic acid are capable of inducing NF-κB and NF-κB target gene expression [24].
Although the lowered ATP generation could be an important factor for the acute decrease in cNOS activity, the intracellular biochemical mechanisms mediating this injury are not completely understood. It has been speculated that unconjugated di- and trihydroxy bile acids cause damage by binding to and crossing cell membranes to enter cells. However, it has recently been shown that the β-chain of ATP synthase, a principal protein complex in the mitochondrial inner membrane, is also present at the cellular surface and plays a decisive role in the regulation of cell homeostasis. At the mitochondrial level, the potential toxicity of bile acids may cause cytochrome C release and Fas-dependent hepatocyte apoptosis or necrosis, and inhibit the activities of complexes I and III of the mitochondrial respiratory chain [25]. However, because of their detergent properties, bile acids are inherently cytotoxic and their direct toxicity to isolated hepatocyte mitochondria has also been demonstrated [26].
Some evidence additionally attests to the importance of direct effects of bile on MCs. It has been shown that lipophilic bile acids possess concentration-dependent cytotoxicity toward MCs, causing histamine release in vitro [3]. Similarly, in vivo bile acids induce secretion in the small intestine by a mechanism involving histamine-mediated processes and MC degranulation [27,28]. However, Fihn et al [29] demonstrated that MC degranulation and NO release are not involved in the mechanism of deoxycholic acid-induced increase in epithelial permeability in rat small intestine [29]. As the secretory effect of conjugated bile acids is observed only in association with an increase in mucosal permeability, a possible explanation could be that a permeability-enhancing factor is necessary to permit access of these charged large molecules to the basolateral membrane or to the subepithelium, where they can then exert their secretory effect. In our study, 7-NI treatment significantly amplified bile-induced tissue damage and caused further MC degranulation. Since 7-NI has a higher affinity for nNOS than for the eNOS isoform [30], the residual responses to 7-NI might be mediated by a previously unblocked part of the nNOS isoform. This also suggests that bile principally inhibits eNOS, and partially sustained nNOS activity is able to counteract, or at least diminish the harmful effects of bile. This underlines the role of nNOS-derived NO in the maintenance of esophageal homeostasis.
Beside increases in blood flow, acute biliary challenge was found to be accompanied with leukocyte recruitment. In many ways, constitutively produced NO is anti-inflammatory, as it modulates the adhesive interaction between leukocytes and endothelium, and reduces MC reactivity. Thus, it could be hypothesized that decreased local ATP level, cNOS inhibition and relative lack of NO could account for the mucosal MC degranulation and the intramural blood flow increase, and these factors may play a concerted role in the process of leukocyte accumulation.
In the PC-pretreated animals, on the other hand, the bile-elicited ATP decrease, cNOS inhibition and MC degranulation were significantly attenuated. The question therefore arises as to which process may be of critical significance in the mechanism of mucosal protection after PC treatment. PC is a ubiquitous membrane-forming entity and the most prominent phospholipid species in the gastrointestinal tract. PC is present in the bile, in vitro and in vivo experiments have demonstrated that topical PC physically protects the intestinal mucosa against injurious actions of bile salts by forming less toxic mixed micelles [31]. Nevertheless, the experimental results and clinical experience suggest that PC could function as an active substance under certain in vivo conditions. The therapeutical effect of dietary PC in preventing esophageal strictures due to alkali-induced esophageal burns has been demonstrated in rats[32], and parenteral PC and lyso-PC could prolong survival in experimental sepsis models[33,34]. PC is taken up by phagocytic cells, and accumulates in inflamed tissues [35]. Little is known about the transport of the molecule, but phosphatidylcholine transfer protein (PCTP) accelerates the intermembrane transfer of phospholipids in an energy-independent way. Choline, a metabolite of PC and a precursor of organic osmolyte betaine, is actively transported by a choline carrier described in intestinal epithelial and endothelial cells of the blood-brain barrier [36].
It is widely believed that the biological efficacy of PC depends on the fatty acid moiety [37]. In contrast, some studies have revealed that the protective role of PC is independent of fatty acids, and it may be assumed that the active principle is choline. Phospholipase-D is activated by almost all stress factors resulting in the release of phospholipid metabolites, and several of these factors could be of importance in stress-induced defense reactions [38]. Indeed, it has been shown that PC metabolites might relieve a potentially dangerous increase in the ratio of NADH/NAD+ (reductive stress), a predisposing cause of oxidative damage [11]. This reaction sequence could explain the still incompletely understood essential role of choline in diet and its preventive efficacy in a number of experimentally induced pathologies associated with a redox imbalance. It may be assumed that the endogenous pool of these metabolites may become exhausted under exogenous provocation and that an exogenous supply might help to replenish and strengthen the endogenous protective mechanism.
In conclusion, our data suggest that nNOS-MC axis plays an important role in the mucosal defense system of the esophagus. Elucidation of the mechanisms by which bile acids induce mucosal dysfunction is complicated by the intrinsic complexity of esophageal tissue, which is made up of many different, but interacting cell types. Whether the findings in this experimental model are applicable to humans remains to be established. However, these data together with previous observations suggest a therapeutic potential for parenteral PC with a view to decreasing the harmful consequences on bile-induced tissue reactions.
The authors thank Dr. Miklos Ghyczy for fruitful discussions, Mr. Armin Wendel (Phospholipid GmbH, Cologne, Germany) for the generous supply of PC, and Drs. László Tiszlavicz and István Németh for the histology analysis.
| 1. | Szentpáli K, Kaszaki J, Tiszlavicz L, Lázár G, Balogh A, Boros M. Bile-induced adenosine triphosphate depletion and mucosal damage during reflux esophagitis. Scand J Gastroenterol. 2001;36:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Schweitzer EJ, Harmon JW, Bass BL, Batzri S. Bile acid efflux precedes mucosal barrier disruption in the rabbit esophagus. Am J Physiol. 1984;247:G480-G485. [PubMed] |
| 3. | Quist RG, Ton-Nu HT, Lillienau J, Hofmann AF, Barrett KE. Activation of mast cells by bile acids. Gastroenterology. 1991;101:446-456. [PubMed] |
| 4. | Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222-G229. [PubMed] |
| 5. | Boros M. Microcirculatory dysfunction during intestinal ischemia-reperfusion. Acta Physiol Hung. 2003;90:263-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Fischer H, Becker JC, Boknik P, Huber V, Lüss H, Neumann J, Schmitz W, Domschke W, Stachura J, Konturek JW. Expression of constitutive nitric oxide synthase in rat and human gastrointestinal tract. Biochim Biophys Acta. 1999;1450:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Qu XW, Wang H, Rozenfeld RA, Huang W, Hsueh W. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine. Regulation by platelet-activating factor. Biochim Biophys Acta. 1999;1451:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Martin GP, Marriott C. Membrane damage by bile salts: the protective function of phospholipids. J Pharm Pharmacol. 1981;33:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | el-Hariri LM, Marriott C, Martin GP. The mitigating effects of phosphatidylcholines on bile salt- and lysophosphatidylcholine-induced membrane damage. J Pharm Pharmacol. 1992;44:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kuehl FA, Jacob TA, Ganley OH, Ormond RE, Meisinger MAP. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J Am Chem Soc. 1957;79:5577-5578. [RCA] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Ghyczy M, Torday C, Boros M. Simultaneous generation of methane, carbon dioxide, and carbon monoxide from choline and ascorbic acid: a defensive mechanism against reductive stress. FASEB J. 2003;17:1124-1126. [PubMed] |
| 12. | Lamprech W, Trautschold I. Adenosine 5’-triphosphate. Determination with hexokinase and glucose 6-phosphate dehydrogenase. Volume 4. New York: Verlag Chemie, Weinheim and Academic Press 1976; 2101-2109. |
| 13. | Szabó C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786-792. [PubMed] |
| 14. | Kuebler WM, Abels C, Schuerer L, Goetz AE. Measurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assay. Int J Microcirc Clin Exp. 1996;16:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Lanas A, Royo Y, Ortego J, Molina M, Sáinz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 342] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | McKie LD, Bass BL, Dunkin BJ, Harmon JW. Nitric oxide regulates basal but not capsaicin-, CGRP-, or bile salt-stimulated rabbit esophageal mucosal blood flow. Ann Surg. 1995;222:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Sandler AD, Schmidt C, Richardson K, Murray J, Maher JW. Regulation of distal esophageal mucosal blood flow: the roles of nitric oxide and substance P. Surgery. 1993;114:285-93; discussion 293-4. [PubMed] |
| 19. | Salvemini D, Maisini E, Pistelli A, Mannaioni PF, Vane JR. Nitric oxide: a regulatory mediator of mast cell reactivity. J Cardiovasc Pharmacol. 1991;17:S258–S264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Szabó A, Kaszaki J, Boros M, Nagy S. Possible relationship between histamine and nitric oxide release in the postischemic flow response following mesenteric ischemia of different durations. Shock. 1997;7:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Rees WD, Rhodes J, Williams BC, Owen E, Newcombe RG. Effect of mast cell degranulation on gastric mucosal damage produced by sodium taurocholate in the rat. Gastroenterology. 1978;74:492-495. [PubMed] |
| 22. | Feldman MJ, Morris GP, Dinda PK, Paterson WG. Mast cells mediate acid-induced augmentation of opossum esophageal blood flow via histamine and nitric oxide. Gastroenterology. 1996;110:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | McKie LD, Dunkin BJ, Pennanen MF, Dunlap KW, Harmon JW, Bass BL. Esophageal mucosal blood flow: a central role for calcitonin gene-related peptide. Surgery. 1994;116:409-17; discussion 417-8. [PubMed] |
| 24. | Jenkins GJ, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, Parry JM. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174-G1188. [PubMed] |
| 26. | Krähenbühl S, Talos C, Fischer S, Reichen J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology. 1994;19:471-479. [PubMed] |
| 27. | Hardcastle J, Hardcastle PT, Chapman J, Taylor CJ. Taurocholic acid-induced secretion in normal and cystic fibrosis mouse ileum. J Pharm Pharmacol. 2001;53:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Gelbmann CM, Schteingart CD, Thompson SM, Hofmann AF, Barrett KE. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J Clin Invest. 1995;95:2831-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Fihn BM, Sjöqvist A, Jodal M. Involvement of enteric nerves in permeability changes due to deoxycholic acid in rat jejunum in vivo. Acta Physiol Scand. 2003;178:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 421] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 31. | Barrios JM, Lichtenberger LM. Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology. 2000;118:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Demirbilek S, Aydin G, Yücesan S, Vural H, Bitiren M. Polyunsaturated phosphatidylcholine lowers collagen deposition in a rat model of corrosive esophageal burn. Eur J Pediatr Surg. 2002;12:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003;44:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Cleland LG, Shandling M, Percy JS, Poznansky MJ. Liposomes: a new approach to gold therapy. J Rheumatol Suppl. 1979;5:154-163. [PubMed] |
| 36. | Friedrich A, George RL, Bridges CC, Prasad PD, Ganapathy V. Transport of choline and its relationship to the expression of the organic cation transporters in a rat brain microvessel endothelial cell line (RBE4). Biochim Biophys Acta. 2001;1512:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Lieber CS, Leo MA, Aleynik SI, Aleynik MK, DeCarli LM. Polyenylphosphatidylcholine decreases alcohol-induced oxidative stress in the baboon. Alcohol Clin Exp Res. 1997;21:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Exton JH. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121-133. [PubMed] |
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M