INTRODUCTION
The spirochete Leptospira interrogans and Borrelia burgdorferi are the etiological agents of leptospirosis and Lyme disease, respectively. All spirochetoses share a bacteriemic phase during the early stage of infection. During this phase, leptospires can cause hepatitis in humans, resulting in microscopic alterations in the liver, including enlargement of Kupffer cells (KCs)[1]. In Lyme disease, liver function test abnormalities are common but mild, most often not associated with symptoms[2], rarely with hepatitis. The causes of hepatitis and abnormal liver function tests in leptospiral and borrelial infections are not known. Circulating levels of tumor necrosis factor α (TNF-α) have been detected in patients with leptospirosis and are associated with the severity of the disease[3]. Although borrelial invasion may cause direct hepatocyte damage, the acute “viral-like” illness associated with early B.burgdorferi dissemination raises the possibility that systemic or local cytokine release as well as other as yet unknown factors may be responsible for hepatic enzyme elevations[2].
The use of in vitro and animal models has shown that spirochetes are phagocytized by KCs, the resident macrophages of the liver[4]. Kupffer cells play an important role in host defense due to their ability to phagocyte bacteria and to detoxify endotoxins[5]. Furthermore, activated KCs play a major role in the initiation and maintenance of liver damage in the above experimental models via production of inflammatory cytokines[6], and trigger a process called the respiratory burst, which involves an increase in cellular oxygen consumption, the production of reactive oxygen species (ROS)[7-10] and nitric oxide (NO) [11]. ROS is cytotoxic for a variety of microorganisms[12], sometimes, however the target of ROS is not limited to the invading pathogens, but can also be extended to host tissues[13], and in the case of the liver, it has been shown that inactivation of KCs prevents liver injury[14]. In this case ROS may cause oxidative damage by peroxidation of membrane phospholipids and alteration in DNA or mithocondrial functions.
Nitric oxide (NO), a gaseous free radical, is a potent biological mediator in a myriad of physiological and pathological events[11]. Abnormalities of NO production have been hypothesized to mediate macrophage cytotoxicity in host defence reactions and to inhibit smooth muscle contraction, hepatocyte metabolism and protein synthesis[15] and to be of central importance in the pathogenesis of many disease processes[16]. Moreover, increased NO production can function as an adaptive response to acute hepatic inflammation and early sepsis[17,18]. NO production is mediated by members of the nitric oxide synthase (NOS) family[19]. The iNOS isoform is usually activated from inflammatory cytokines and bacterial products, and is responsible for generating high levels of NO[11].
In this study we analyzed the interaction of live L. interrogans and B. burgdorferi with isolated rat KCs in vitro, as far as the production of ROS and iNOS expression is concerned.
MATERIALS AND METHODS
The following spirochete strains were used: B. burgdorferi IRS (ATCC 35211) and L. interrogans serovar icterohaemorrhagiae (a gift of M. Fabbi, Istituto Zooprofilattico Sperimentale, Pavia, Italy). Borreliae were cultured in Barbour-Stoenner-Kelly (BSK) II medium at 34 °C, as previously reported[20], whereas leptospirae were grown in liquid Ellinghausen-McCullough-Johnson-Harris (EMJH) medium at 30 °C under aerobic conditions to a density of about 108 bacteria per mL and counted in a Petroff-Hausser counting chamber.
When indicated, opsonized bacteria were prepared as follows: spirochetes were centrifuged and resuspended in RPMI 1640 at a concentration of 4 × 107 bacteria/mL. Bacterial suspensions were incubated for 30 min with pooled sera obtained from Lyme disease and leptospirosis patients, at a concentration of 10%. After incubation, spirochetes were washed twice in RPMI 1640 to remove non-haderent antibodies.
Kupffer cells (KC) were harvested and separated following the procedure of Smedsrød and Pertoft[21] with some modifications, as previously reported[4]. Male Sprague-Dawley rats (180 g to 200 g body weight) were used as liver donors. The animals were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally), and the livers were perfused through the portal vein, with 500 mL of calcium- and magnesium-free Hanks’ balanced salt solution, which was pre-warmed to 37 °C and buffered at pH 7.3 with 10 mol/L HEPES (Sigma Chemicals, St. Louis, MO, USA). The effluent from the liver was collected from the inferior vena cava immediately above the sovrahepatic veins. The liver was then perfused with complete Hanks’ balanced salt solution containing 0.05% collagenase (type IV; Sigma), pre-warmed to 42 °C, and subsequentely excised and placed in a Petri dish. The liver capsule was peeled off and the cells were gently dispersed in calcium- and magnesium-free Hanks’ balanced salt solution. The cells were then centrifuged at 50 r/min at 4 °C for 2 min in a Beckman J6B centrifuge (Beckman Instruments, Palo Alto, CA, USA). The non-parenchymal cell-enriched supernatant was centrifuged at 800 r/min for 10 min, the pellet was resuspended in 40 mL of phosphate-buffered saline (PBS), and portions of 10 mL were layered on top of a preformed two-step Percoll gradient, and centrifuged at 400 r/min for 15 min at 4 °C. Purified non-parenchymal cells enriched in KCs extended throughout the lower Percoll cushion. The KC-enriched fraction was diluted in PBS and centrifuged at 800 r/min for 10 min. The resulting pellet was resuspended in culture medium (RPMI 1640 with 10% fetal calf serum) at a concentration of 1.0 × 106 cells/mL. A 0.5 mL portion of cell suspension was added to an 8-well culture plate (LAB-TEK, Nalge-Nunc International, Naperville, Ill). Macrophages were selected by allowing them to adhere for 2 h at 37 °C, in an atmosphere with 50 mL/L. After non-adherent cells were removed by gentle washing, adherent cells were incubated with RPMI 1640 for 24 h before performing further experiments. As a control, immunofluorescence staining was performed with mouse anti-rat CD11b antibody (Research Diagnostics Inc, Flanders, NJ, USA), more than 95% of adherent cells were positive for CD11b macrophage marker.
Reactive oxygen species produced by KCs was determined by chemiluminescence (CL). The determination of ROS was performed as previously reported[6,10]. Briefly, 0.25 mL of a suspension of purified KCs was added (2 × 106 cells/mL) to selected wells (see below) of a transparent 96-well microtiter plate (Nunclon, Nunc, Denmark). Macrophages were selected by allowing them to adhere for 2 h at 37 °C, in an atmosphere with 50 mL/L CO2. After non-adherent cells were removed by gentle washing, adherent cells were incubated with RPMI 1640 overnight before performing the assays. The culture medium was replaced with 200 μL of a fresh one, and 50 μL of a 3 mmol/L luminol solution (5-amino-2,3-dihydro-1,4-phthalazinedione sodium salt, Sigma) in TRIS buffer (0.1 mol/L, pH 7.4) was added to each well followed by 50 μL of a spirochete suspension in culture medium (final ratio 200-2000 bacteria/cell). All reagents were pre-warmed at 37 °C before addition. The final luminol concentration was 0.6 mmol/L. Each experiment was performed in triplicate. In order to normalize the CL signal for variables as the number of KCs and cell viability, Kupffer cells stimulated by a fixed concentration (0.8 μmol/L) of phorbol myristate acetate (Sigma) were used as positive control in each run. For each experiment, the background signal of the CL cocktail was evaluated by measuring blank samples constituted by Kupffer cells in growth medium.
The microtiter plate was placed in a luminometer (Luminoskan Ascent, Labsystems, Wallac Oy, Finland) at 37 °C, and the CL emissions expressed as relative light units were acquired at 2 min intervals for 3 h, using an integration time of 10 s. Quantitative evaluation of the signals was performed by evaluating the integrated CL signal over the whole measurement period. Then, the CL response of the system was expressed as the ratio between the integrated CL signal of the sample and that of the control. Additional control experiments, in which no luminol was added, were performed. Use of a microtiter plate format allowed to simultaneously follow the kinetics of the chemiluminescent emission for different samples in a simple way. Due to the possible cross-talk between adjacent wells, no more than 12 samples were measured in the same analytical session. Separate experiments carried out with a conventional chemiluminescent system (horseradish peroxidase, luminol, hydrogen peroxide) confirmed that for the emission intensities found in our experiments the cross-talk between different wells was negligible (less than 0.1%) if the wells were separated by at least two empty ones. Mean values of ROS production by KCs were compared using Student’s t-test. P < 0.01 was considered statistically significant.
The determination of iNOS expression by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting was performed as follows. KCs isolated as reported above, were incubated with 200 spirochetes/cell, re-suspended in RPMI 1640, opsonized and unopsonized up to 22 h. As positive controls LPS (10 μg/mL) and INF-γ (100 UI/mL) were used, whereas as negative control any stimuli were omitted. Three different time points were evaluated: 2, 6 and 22 h. At the end of each time point, KCs were scraped, diluted with sample buffer (20% vol/vol Tris 0.5 mol/L, pH 6.8, 2% w/vol SDS, 20% vol/vol glycerol, 10% vol/vol 2-mercaptoethanol, and 0.02% w/vol bromophenol blue) and sonicated. Protein concentration was determined by the Bradford assay. Separation of KC proteins (5 μg/each lane) was performed with a Laemmli buffer system by using a 12% acrylamide gel, as previously described[22]. The Western blot procedure was performed as previously described[23]. Briefly, after electrophoretic transfer, the blots were incubated overnight at room temperature with polyclonal rabbit anti-iNOS antibodies (Affinity Bioreagents, Golden, CO, USA) diluted 1:100 in PBS containing 0.05% (vol/vol) Tween 20. Antigen-antibody complexes were detected using peroxidase-labeled porcine antibodies to rabbit immunoglobulins (DAKO, Copenhagen, Denmark) diluted 1:100 in PBS-Tween. The apparent molecular weight of each band was determined by plotting the positions of rainbow low molecular weight standards (Amersham Biosciences, Little Chalfont, UK). Blots were scanned and the intensity of iNOS bands was determined capturing the images with a Kodak 120 Zoom Digital Camera (Eastman Kodak Company, Rochester, New York, USA) and image processing was performed with Kodak Digital Science 1D 3.0.2 (Eastman Kodak Company, Rochester, New York, USA).
RESULTS
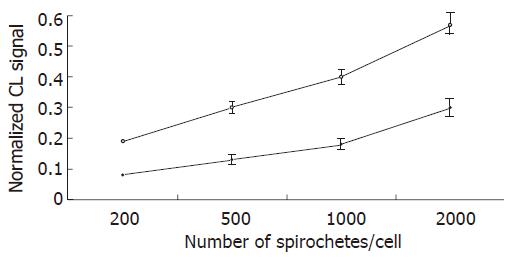
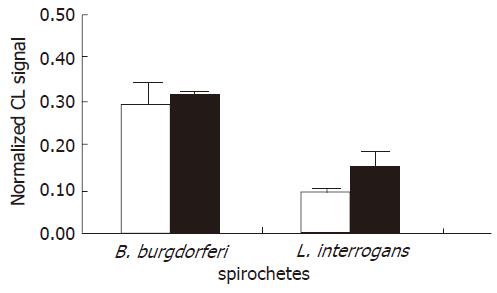
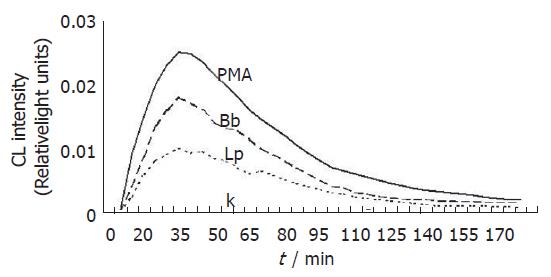
To study ROS production by KCs following incubation with spirochetes, the liver macrophages were incubated alone or with different numbers of spirochetes, and the production of ROS was determined by chemiluminescence. Spirochetes induced ROS production in KCs in a dose-dependent manner (Figure 1). Opsonization of spirochetes increased ROS production, but the increase was not statistically significant (Figure 2), in addition the ROS production induced by borreliae was about twice the generation of oxygen radicals induced by leptospirae (P < 0.01) (Figure 2). The time course study of oxygen radical generation showed that ROS was detectable as early as 20 min after the beginning of infection. Leptospirae and borreliae both induced ROS production with a peak 35 min after the addition of spirochetes. The chemiluminescence signal progressively diminished following incubation time and was undetectable for all spirochete preparations at 180 min of incubation (Figure 3). Further time point determinations (4, 6 and 8 h) did not show any ROS production (data not reported).
Figure 1 Chemiluminescence (CL) detection of reactive oxygen species generation by Kupffer cells stimulated by L.
interrogans and B.burgdorferi. Kupffer cells were incubated in triplicate with borrelia (white circles) and leptospira (black circles) preparations at various bacteria/KC ratio, for 3 h in the presence of 0.6 mM luminol. The chemiluminescence emissions were acquired at 2 min intervals for 3 h. The experiments were repeated three times, and data points represent the mean ± SD of the integrated chemiluminescence signal, normalized to that of the control.
Figure 2 Effect of spirochete opsonization on the chemiluminescence (CL) signal.
Each value represents the mean ± SD of three independent experiments carried out at a ratio of 500 spirochetes/KCs using unopsonized (white bars) and opsonized (grey bars) bacteria. Note that the ROS production induced by borreliae was about twice the ROS generation of leptospirae.
Figure 3 Chemiluminescence kinetic profiles of reactive oxygen radical generation in Kupffer cells treated with L.
interrogans and B.burgdorferi. Bacteria were used at a ratio of 2000/KCs. Lp and Bb stand for leptospirae and borreliae, respectively. PMA represents Kupffer cells treated with phorbol myristate acetate, whereas K represents untreated control Kupffer cells.
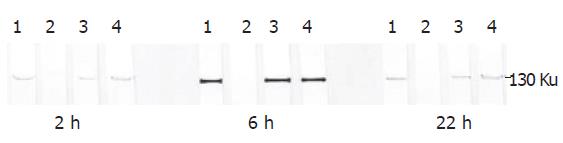
To determine whether spirochetes could modify iNOS expression in KCs, iNOS protein was looked for by Western blot assay using polyclonal anti-iNOS antibodies that specifically detect a band of molecular weight of 130 Ku[24]. Kupffer cells were collected 2, 6 and 22 h after bacterial stimulation at the ratio of 200 bacteria/cell. Untreated Kupffer cells as well as LPS- and interferon-γ- stimulated Kupffer cells were also studied. The protein content of each sample was determined and samples of 5 μg of protein content were run in each lane and separated by SDS-PAGE. The proteins were then blotted and tested with specific anti-iNOS antibodies. The blots were then scanned and the intensity of iNOS bands was determined capturing the images. The iNOS protein band was evident 2 h after infection. The band peaked 6 h after infection and was still evident 22 h after infection. Six hours after infection, the iNOS band was 5 and 2.5 times more intense than that 2 h and 22 h after infection, respectively. The iNOS protein band was also induced by LPS plus INF-γ (positive controls), whereas the iNOS was not detectable in the absence of stimulating agents (Figure 4). No evident differences in the expression of iNOS protein were seen between KCs stimulated with B. burgdorferi and L. interrogans, both of which were opsonized and unopsonized.
Figure 4 iNOS determination by SDS-polyacrylamide gel electrophoresis and by Western blotting in Kupffer cells stimulated by L.
interogans and B. burgdorferi. The 130 ku iNOS protein band was detected by specific polyclonal rabbit anti-iNOS antibodies. The iNOS protein band was evident 2 h after infection. The band peaked 6 h after infection and was still evident 22 h after infection. Lane 1: Kupffer cells stimulated by LPS and interferon-γ (positive control); lane 2: Kupffer cells not stimulated (negative control); lane 3: Kupffer cells stimulated by L.interrogans; lane 4: Kupffer cells stimulated by B.burgdorferi.
DISCUSSION
The macrophages of the liver, referred to as Kupffer cells are the most abundant mononuclear phagocytes in the body. The major function of KCs is to clear particulate and foreign materials and micro-organisms from the blood. When activated by antigens or inflammatory stimuli, KCs release superoxide anions, hydrogen peroxide, nitric oxide and hydrolytic enzymes. Kupffer cells also release a number of different immunoregulatory and inflammatory cytokines, including interleukin (IL-1), IL-6, tumor necrosis factor α, interferon-γ[25,26].
Leptospirae and borreliae are phagocytosed by the Kupffer cells in the absence of opsonizing antibodies (Marangoni et al personal Communication VIII International Conference on Lyme borreliosis, Munich, 20-22 June, 1999). The pro-inflammatory cytokine TNF-α is produced by Kupffer cells treated with spirochetes and leptospirae have about twice the stimulatory effect of borreliae[6]. Leptospiral lipo-oligo-saccharide plays a pivotal role in the TNF-α release[6].
The generation of ROS by macrophages and the expression of iNOS occur during the phagocytosis of bacteria, fungi and protozoa[27-29]. In the present work, we comparatively studied the ability of KCs to generate ROS and to express iNOS when stimulated by L. interrogans and B. burgdorferi. Our findings showed that both the genera of spirochetes induced ROS production in KCs. The use of such a very sensitive method as CL allowed us to demonstrate that when the same amount of bacteria was employed ROS production induced by borreliae was about twice the generation of oxygen radicals induced by leptospirae. This could be partly due to the different size of the bacteria, B. burgdorferi being longer and wider than L. interrogans. The production of ROS had a maximum 35 min after infection and was undetectable 180 min after infection. The transient feature of the oxidative burst is well documented in monocytes and macrophages, in particular, a similar short-lived and self-limiting oxidative response has also previously been observed during phagocytosis of Salmonella by mouse peritoneal macrophages[30,31] and during the phagocytosis of T. pallidum by Kupffer cells[9]. In this study, opsonized leptospirae as well as opsonized borreliae did not enhance ROS production by Kupffer cells in comparison with non-opsonized bacteria. In general, opsonization enhances phagocytosis of bacteria and consequently the production of ROS. However, it has been previously demonstrated (Marangoni et al personal Communication VIII International Conference on Lyme borreliosis, Munich, 20-22 June, 1999) that opsonization of leptospirae and borreliae does not enhance phagocytosis by Kupffer cells. Kupffer cells phagocytose leptospirae and borreliae at the same rate in the presence and in the absence of opsonizing antibodies. This probably comes from a constitutional attitude of these bacteria that are not able to escape phagocytosis, differently from T. pallidum, a spirochete able to evade phagocytosis by macrophages to a certain extent.
Regarding iNOS, we established that both borreliae and leptospirae induced iNOS expression by Kupffer cells and observed no substantial differences between the two genera of spirochetes in inducing iNOS production.
iNOS is regulated at the transcriptional level of the gene and once induced, iNOS production is maintained for several hours or days[24]. Kupffer cells produce nitrite and nitrate, the end stable products of NO pathway and the overproduction of NO by hepatic cells play a major role in hepatic injury and necrosis[32] associated with endotoxic shock. Kupffer cells also synthetize several cytokines in response to LPS which in turn stimulate the hepatocytes to produce NO[33].
The present in vitro investigation demonstrated that both borreliae and leptospirae could induce iNOS in rat Kupffer cells and that iNOS production was delayed with respect to ROS production. The in vivo exposure of KCs to these bacteria, as in the case of leptospirosis and Lyme disease, could lead to a maintained production of NO. In fact, iNOS produces NO from hours to days and overproduction of NO can stimulate neighbouring hepatocytes to generate NO[28] in the liver, resulting in the production of NO over a long period of time after exposure of KCs to leptospirae and borreliae.
In conclusion, our data suggest that a factor responsible for hepatic enzyme elevations in leptospirosis as well as in Lyme disease might be a sequential mechanism of damage initiated by oxygen radicals produced in KCs and maintained by NO production in KCs and likely in hepatocytes.
S- Editor Wang J L- Editor Wang XL E- Editor Zhang Y