INTRODUCTION
Although the phenomenon of mental disturbance in patients with disordered liver function was probably first described over 2000 years ago by Hippocrates, the precise mechanisms associated with the development of hepatic encephalopathy (HE) remain unclear. The syndrome occurs in two distinct forms: in the context of acute liver failure, acute HE results in impaired consciousness which may progress to coma and death over a period of hours or days, whereas chronic HE describes the neuropsychiatric syndrome, most commonly associated with hepatic dysfunction and portosystemic shunting in cirrhosis. This may also exist in patients with surgical portosystemic shunts[1]. In most patients the condition is “minimal” and, on a functional basis, it is discernible in prolongation of reaction times in the activities of daily living, such as driving and operating machinery, but in about 30% of patients with chronic liver disease, the syndrome is more severe with alterations in behavior, mood, personality and disturbances in consciousness[1].
In routine clinical practice, brain imaging in the form of a CT scan has been commonly used to exclude other diagnoses, such as subdural hematomata, which may also cause impaired consciousness in patients with chronic liver disease. Until recently, the use of other imaging modalities such as magnetic resonance imaging (MRI) has been a second-line investigation in many countries, whereas magnetic resonance spectroscopy (MRS) has been largely confined to research applications, where significant advances in the understanding of pathophysiological mechanisms have been made. While most clinical MRI systems have operated at magnetic field strengths of 0.5-1.5 Tesla (T), higher field strength whole body systems are now increasingly becoming available at 3T and above, providing increased resolution and sensitivity. This article will review the published MR studies in HE and with the advent of new MR imaging capabilities, we speculate on areas of new research for the future.
HEPATIC ENCEPHALOPATHY
“Minimal” HE is the term used to describe the presence of cognitive impairment on psychometric testing and/or slowing of electroencephalographic (EEG) mean cycle frequency in the absence of any clinically overt signs[1,2]. This form of HE is present in the majority of patients with cirrhosis, but where the condition is more profound, it is usually characterised by remissions and relapses, manifested by obvious mood disorders, personality changes and disturbances in consciousness[1]. However, in a small minority of patients the neuropsychiatric impairment may follow a more persistent course. Elevated circulating ammonia levels, which are present owing to impaired hepatic nitrogen excretion or because of portosystemic shunting, are thought to be key to the changes in neurotransmitter systems that underlie the disturbances in function observed clinically[3]. These include increased GABA-ergic tone, elevated glutamine levels and changes in several other neurotransmitter systems and their receptors, such as reduced glutamate excitatory transmission and increased serotonergic inhibition[4]. Other abnormalities include selective alterations of blood-brain barrier permeability and changes in cerebral energy metabolism[5]. However, the central question of how these relate to elevated ammonia concentrations is an unresolved issue.
MAGNETIC RESONANCE IMAGING
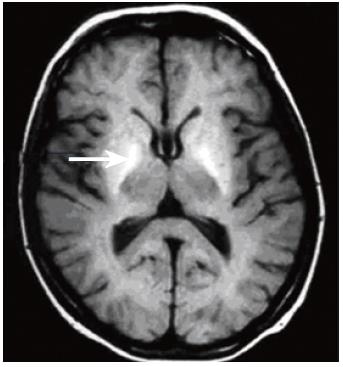
CT and MRI have long been used to detect obvious structural abnormalities in the brain such as cerebral atrophy, which occur in patients with chronic alcoholism, who do not necessarily have significant liver disease[6,7]. However, MR techniques may provide non-invasive insight into the pathogenesis of HE. Apart from structural changes associated with the direct effects of alcohol, a longstanding MR observation has been that patients with cirrhosis of any aetiology show hyperintensity in the basal ganglia on T1-weighted MRI (Figure 1)[8-11]. Hypermanganesemia due to occupational exposure or total parenteral nutrition also results in similar appearances, which reverse after correction of the blood manganese levels[12,13]. In cirrhosis, a correlation between blood manganese levels and globus pallidus signal intensity has been reported[10,14,15] and elevated post-mortem globus pallidus manganese concentrations have been detected in patients who have died with terminal liver disease[10,16]. It was initially suggested that basal ganglia hyperintensity might be an important aetiological association of the form of HE that complicates chronic liver disease[8,11]. However, a number of studies found no correlation between basal ganglia T1-weighted signal intensity and clinical encephalopathy or neuropsychological test performance[9,17,18], although the extrapyramidal symptoms seen in a minority of patients with HE may be related[14]. Instead, basal ganglia T1-weighted signal intensity and manganese accumulation appears to be related to the underlying degree of hepatic dysfunction or more particularly to the severity of cholestasis and/or the presence of portosystemic shunting, rather than directly to neuropsychiatric impairment[19]. The relationship with cholestasis is important, since manganese itself is normally eliminated from the body via the biliary route[20].
Figure 1 T1-weighted brain MR image through the basal ganglia acquired at 1.
5T, showing bilateral globus pallidus (arrow) hyperintensity in a patient with cirrhosis of the liver.
It has recently been proposed by Häussinger and colleagues that a major contributory event in the development of HE in patients with chronic liver disease is an increase in astrocyte hydration, or in other words, a low-grade cerebral oedema without a clinically overt increase in intracranial pressure[5]. This may occur as a consequence of ammonia uptake by astrocytes and subsequent detoxification by the enzyme glutamine synthetase to form glutamine from glutamate[3]. This deamidation process is thought to result in the accumulation of glutamine within astrocytes, which acts as a cerebral osmolyte with resultant cell swelling. With conventional neuroimaging, brain oedema is not evident in the vast majority of patients with chronic liver disease who have HE, probably because if present, the brain swelling is minimal and is thus beyond the threshold for detection on standard MRI. This is unlike the encephalopathy associated with fulminant hepatic failure, which is obviously characterised by cerebral oedema [21]. However, a number of recent studies, which employed magnetization transfer imaging (MT), suggested that there may be an increase in total brain water in the HE of chronic liver disease[22-24].
MAGNETIZATION TRANSFER IMAGING
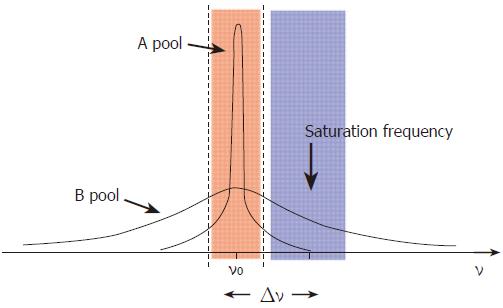
MT allows indirect measurement of bound and free water compartments in the brain and may be affected by changes in membrane fluidity, heavy metal deposition and alterations in total water content[23,24]. The technique itself is a method for manipulating tissue contrast[25,26]. In addition to enabling the acquisition of enhanced contrast images, techniques employing MT also allow the measurement of MT ratios (MTR). The MTR is a quantitative tissue characteristic that reflects the behavior of normally MR-invisible protons bound to macromolecules. MTR measurement may detect brain parenchymal changes that may not be visible using standard MR techniques. In essence, protons in tissues can be described as existing in two pools. The free pool consists of mobile protons, such as those in body water, and has a narrow spectral line with relatively long T1 and T2 relaxation times (Figure 2). The bulk of the signal in conventional MR systems comes from this free pool, as the MR excitation frequency range is narrow and centered upon these freely mobile protons. A second pool of protons bound in proteins, other macromolecules and membranes, is conventionally said to be MR invisible, as it falls outside the typical excitation range. This pool has a broad spectral line and very short relaxation times (Figure 2). Magnetization can be transferred bi-directionally between pools by direct interaction between spins, the transfer of nuclei or by direct chemical means. Under normal circumstances, the transfer of magnetization is the same in both directions.
Figure 2 Model demonstrating the concepts underlying the phenomenon of magnetization transfer.
The free pool of protons (A) resonates at the Larmor frequency (ν0) and has a narrow spectral line. It is excited by an RF pulse which covers the frequencies shown in pink. The “bound” pool of protons (B) has a broad spectral line and can be excited and saturated by the application of RF irradiation at a frequency offset by Δν, shown in blue, without significantly affecting pool A.
Techniques that employ MT do so by saturating the magnetization in the bound pool while leaving the free pool largely unaffected. This is possible because the bound pool has a broad spectral line and can be excited by the application of an “off-resonance” RF pulse (Figure 2). This saturation results in substantial attenuation of the magnetization in the bound pool. As a consequence, there is little transfer of magnetization to the free pool and both the effective longitudinal magnetization within it and its T1 are reduced. Pulse sequences that incorporate the application of an “off-resonance” pulse can be designed to allow quantification of the effect of MT in different tissues, in health and disease[25]. Reduced MTRs may result from pathologies that alter the structural integrity and the relative macromolecular-water composition of brain parenchyma, such as in multiple sclerosis plaques or in end-stage cirrhosis[27-29]. These observations may also result from the deposition of paramagnetic substances in the brain. MR experiments with manganese chloride phantoms have demonstrated a strong inverse linear relationship between the manganese concentration and the MTR[29]. This property has been exploited for the use of paramagnetic contrast agents, such as gadolinium-based compounds. Furthermore, manganese is excreted via the biliary route and can thus accumulate in cholestatic conditions, including in the basal ganglia in patients with chronic liver disease[19]. It is of note, therefore, that our group has reported reduced MTRs in the basal ganglia of fatigued patients with primary biliary cirrhosis, which correlate strongly with ambient plasma manganese levels[30].
In the context of HE, the reported change in MTR in patients with cirrhosis could represent an alteration in the structure of cell membranes, leading to cell membrane fluidity. However, these observations may, perhaps, be accounted for by an increase in cerebral water content[22-24]. If the interpretation of the MT papers in HE is correct[22-24], an increase in brain hydration, as represented by altered MT ratios, will not be dramatic enough to cause an increase in intracranial pressure, but may reflect “lower-level” astrocyte swelling, which could be sufficient enough to trigger alterations in astrocyte function[22-24]. The possible functional effects of low grade astrocytic swelling are numerous and could possibly explain many of the features of HE complicating chronic liver disease, including alterations in blood brain barrier permeability, neurotransmitter processing and receptor density and upregulation of cerebral neurosteroid production[5]. At present, the MTR data remain an interesting observation and future studies incorporating newer MR imaging techniques are required to provide more definitive answers on pathogenesis.
DIFFUSION IMAGING
Diffusion-weighted imaging (DWI) is a method used to quantify the movement of water molecules. One of the first applications of this technique was the detection of acute cerebral ischemia in the early 1990s[31,32]. Further recognized indications include the investigation of multiple sclerosis[33] and brain tumors[34,35].
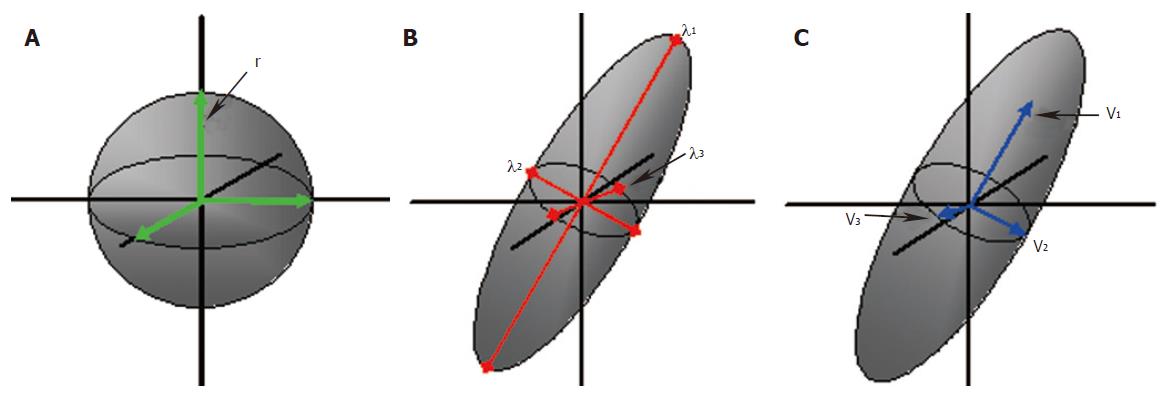
The diffusion of water molecules follows the principles of Brownian motion. Thus, when these molecules are unconstrained, their movement is random and therefore equal in all directions, being described as “isotropic”. However, the motion of water molecules in structured environments is restricted, according to the physical surroundings. The motion of water molecules in the brain is restricted by the microstructure within grey and white matter. Water molecules on average tend to move parallel to, rather than perpendicular to the white matter tracts[36,37]. This motion is described as “anisotropic”, as it is no longer equal in all directions. The motion of the molecules in the x, y and z directions and the correlation between the directions is described with the diffusion tensor[38,39]. A tensor is a mathematical construct that defines the properties of a three-dimensional ellipsoid. To define the diffusion tensor requires acquisition of diffusion data in a minimum of six non-collinear directions, this is known as diffusion tensor imaging (DTI). The diffusion tensor is graphically represented by a three-dimensional ellipsoid, with the long axis representing the primary direction of motion (Figure 3). Eigenvalues and eigenvectors describe the magnitude and directions of the three principal axes of the diffusion ellipsoid[40]. The diffusion tensor is evaluated at each pixel location and so maps of the magnitude and dominant direction of diffusion can be produced. The dominant directions can be followed from one pixel to another, plotting lines along which diffusion is likely to occur. This practice is known as tractography, the name coming from the implication that these paths are believed to represent white matter tracts. Using a minimum of 2 differently weighted DWI images, a measure of diffusion can be calculated. These data can then be mapped to create an apparent diffusion coefficient (ADC) image[41]. The ADC is a measure of tissue water diffusivity and is dependent upon interactions between water molecules and their surrounding chemical and structural environment[42]. Diffusion data may be expressed in terms of the mean diffusivity, sometimes called the mean ADC or the trace ADC. This is the average of three ADC measurements in three orthogonal directions. DTI gives rise to detailed information on the microstructure within an imaging voxel, including mean diffusivity, the degree of anisotropy and the direction of diffusivities[43]. The mean diffusivity reflects the displacement of water molecules and the presence of obstacles at both the cellular and subcellular level. Fractional anisotropy (FA) and relative anisotropy (RA) are commonly used to express the degree of anisotropy. Anisotropy will be related to the characteristics of any physical barriers, such as the orientation, density, size and shape of nerve fibers within white matter tracts. However, interestingly, myelination has been demonstrated not to be a primary determinant of anisotropy, but may indeed have modulatory effects[44]. The directionality of fibers can be expressed on color-coded two-dimensional maps, or by three-dimensional tractography images. The orientation of the major axonal fiber bundles can be calculated using the eigenvectors and eigenvalues by various algorithms[40]. The expression of DTI data in the form of 3D images is one of the latest developments in this technique and may provide insight into defects in brain connectivity (Figure 4).
Figure 3 Principles of diffusion: A water molecule in an unconstrained environment would move randomly and equally in all directions; isotropic diffusion (A).
The probability of motion can be defined by the radius ‘r’, of the spherical range of motion. Diffusion in an ordered environment such as the white matter will assume an elliptical range of motion (B and C). Three eigenvalues, λ1, λ2 and λ3 define the shape and three eigenvectors v1, v2 and v3 define the orientation of the ellipsoid.
Figure 4 Color fractional anisotropy (FA) image from a 44 year-old healthy male volunteer.
Diffusion tensor imaging (DTI) performed on a 3T Philips InteraTM using 32 different directions of diffusion sensitization. The different colors represent the principle diffusion directions and hence the direction of the white matter tracts: green represents anterior-posterior, blue represents caudo-cranial, red represents transverse. FA maps were generated using DTI studio version 2.1©, (Jiang and Mori, Johns Hopkins University, MD, USA).
In the field of HE, one study has looked at regional brain ADC values in patients with cirrhosis, secondary to viral hepatitis[45]. Seven patients had no encephalopathy, six had grade I and one grade II hepatic encephalopathy, according to Parsons-Smith criteria[46]. The authors found that except in the thalamus, ADC values were significantly increased in all white matter and deep grey matter areas examined. Furthermore, except for the caudate nucleus, there was a positive correlation between ADC values in all areas of the brain and venous ammonia concentrations. They suggest that their findings are related to increased brain water content, which would support the brain swelling hypothesis.
FUNCTIONAL MRI
It is well recognized that HE encompasses a broad spectrum of neuropsychiatric and cognitive impairments. Investigators have used a variety of non-invasive techniques, such as electroencephalography (EEG)[47] and magnetoencephalography (MEG) [48] to study the brains of patients with chronic liver disease, but these methodologies suffer from limited spatial resolution. Positron emission tomography (PET) has also been used to investigate both metabolic impairments and neurotransmitter deficits in patients with HE[49-52], but PET does not readily lend itself to serial studies, owing to the necessity for injection of radioactive tracers.
However, functional MRI (fMRI) provides the opportunity to study brain function in detail without the drawbacks of those other techniques, albeit with a reduced sensitivity resulting in long scan times. The most common method of assessing an alteration in cerebral activity in fMRI is by measuring the “blood oxygenation level dependent” (BOLD) signal, which represents local changes in hemoglobin oxygenation due to cerebral blood flow changes[53-55]. The signal arises because of the different magnetic properties of oxygenated hemoglobin, which is isomagnetic, and deoxyhemoglobin, which is paramagnetic relative to tissue[56]. PET studies have previously demonstrated that cerebral blood flow increases by up to 50% in response to functional activation, but the cerebral metabolic rate for oxygen increases only by 5%[57,58]. Thus, in fMRI there is a reduction in the proportion of deoxyhemoglobin, and hence in the magnetic properties of the activated brain region, which contributes to the BOLD signal. In the context of chronic liver disease, one study has used fMRI to investigate cognitive alterations in a cohort of 9 patients with minimal or “non-manifest” hepatic encephalopathy (nmHE)[59]. The patients were given a stimulus of a flickering light of decreasing frequency and were asked to indicate when the light appeared to change from a fused light to a flickering light; the “critical flicker frequency” threshold[60]. The BOLD signal was measured during this task, and the investigators observed a decrease in activation in the right inferior parietal cortex of the nmHE patients, compared to healthy age-matched volunteers. These data also suggested impaired neural connections between several cortical structures[59].
CEREBRAL BLOOD FLOW
It is well known that cerebral blood flow (CBF) changes in encephalopathic patients[49,61]. There are a variety of MRI techniques that can potentially be used to study this, including arterial spin-labeling (ASL) and contrast-enhanced perfusion MRI (CEMRI)[62-64]. Perfusion can be calculated by measuring the change in signal in the brain as a contrast agent (Gd-DTPA) flows into the brain. If an accurate measure of the arterial input function (AIF) can be extracted then quantitative measures of blood volume, flow and transit time can be calculated. Accurate measurement of the AIF is difficult and so absolute quantification is rare. In addition, the use of contrast agents is not desirable in patients with poor liver function. As always with contrast agents, longitudinal studies are also not recommended which explains the lack of such studies to date. An emerging methodology is arterial spin labeling where the blood is used as an endogenous contrast agent. The blood is tagged (usually in the carotid artery) using an RF pulse, similar to those used in standard MR imaging. The arrival of this “bolus” of tagged blood is then monitored in the brain by imaging. ASL is a very low sensitivity method compared to CEMRI and so requires significantly longer imaging times to acquire robust data (10-20 min compared to < 1 min for CEMRI). This means that ASL really only becomes feasible at higher field strengths (3T+), where imaging times become acceptable due to increased sensitivity. At the time of writing this methodology has yet to be applied to the study of HE, but the technology is now at a level of development that the known CBF changes associated with HE may be able to be quantified and response to treatment tracked using such methods.
VOLUMETRIC MRI
Until recently, MR measurement of brain and ventricular volumes was not sensitive enough for direct, serial measurement of small changes in brain size in patients with HE, who have structurally normal brains. However, the rapid development of MR analysis software now allows for precise measurements of whole or regional brain volumes and allows very small differences in brain size to be monitored that may not be visible to standard radiological inspection (coregistered MRI)[65,66]. These volume changes may be measured to allow inter- or intra-group comparisons and to follow up individuals or groups longitudinally. The techniques have been applied to a number of clinical conundra including pre-eclampsia[67-70] and given the recent interest in the possibility of low-grade oedema as a central factor in chronic HE[5], a useful application of MR is to investigate the brain volume changes that may occur in HE. One such study was performed by our group[71]. Six patients had minimal HE and three had overt HE. Seven patients were treated with lactulose for 6 weeks and three of them improved on serial psychometric testing with a consequent reduction in brain size on repeat MR imaging. Although measurable changes were found after treatment intervention, these were not correlated with the degree of encephalopathy. A recent study used voxel-based morphometric analysis of MR data to calculate brain volumes in patients with cirrhosis of varying etiologies[72]. It is not clear whether these patients were formally assessed for the presence of encephalopathy. However, the investigators found that patients with alcoholic cirrhosis had atrophy involving both grey and white matter structures[72], the latter finding in white matter is consistent with early neuropathological studies[73]. Interestingly these authors also found that patients with cirrhosis, caused by viral hepatitis also had similar, but quantitatively less atrophy[72].
LOCALIZED BRAIN WATER CONTENT
One imaging group has developed an MR technique to measure localized cerebral water content in a quantitative fashion[74,75]. This technique has been used to investigate a cohort of patients with hepatic encephalopathy[76]. Results from this pilot study show a significant correlation between the severity of HE and white matter water content. This could be a technique of great interest, if it can be translated to other MR systems in other centers around the world.
MAGNETIC RESONANCE SPECTROSCOPY
Further evidence for changes in brain hydration and cerebral osmolytes in HE which complicates chronic liver disease comes from studies using MRS. In vivo MRS is a non-invasive technique which can be used to provide localized biochemical information on cerebral metabolic processes. The underlying principles of physics that govern MRS are essentially the same as for MRI. Nearly all elements possess at least one naturally occurring isotope amenable to MR study. The most commonly used isotopes have been hydrogen-1 (1H), and phosphorus-31 (31P). 1H MRS can be utilized to provide information on brain metabolites such as choline, creatine, N-acetyl aspartate (NAA), glutamine and glutamate, as well as osmolytes such as myoinositol and taurine[77].
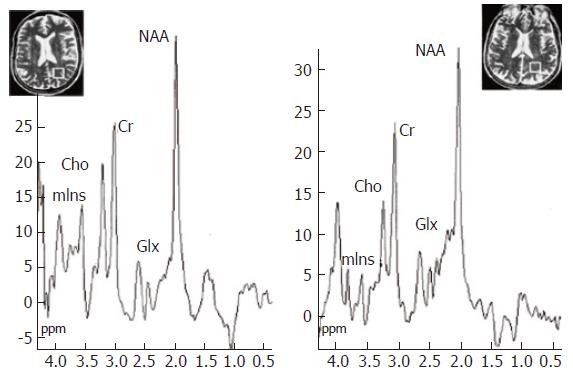
Several studies have reported characteristic spectral appearances in HE, typified by a reduction in the myoinositol and choline resonances and an increase in the glutamate/glutamine composite resonance, which correlate with neuropsychiatric impairment (Figure 5)[77-85]. Ammonia itself cannot be detected by 1H MRS, but the increase in signal from the glutamine/glutamate is likely to reflect ammonia incorporation into glutamine. The astrocyte swelling hypothesis predicts that homeostatic mechanisms occur to limit the osmotic load in astrocytes, leading to the extracellular release of osmolytes, such as myoinositol[5]. This is supported by the observation of reduced intracellular myoinositol in 1H MRS studies.
Figure 5 1H MR spectroscopy water-suppressed proton spectra of an 8 mL voxel located in the parietal region including predominantly normal appearing white matter (insert images show position of the voxel) in a healthy control subject (left) and in a cirrhotic patient (right), acquired using a STEAM pulse sequence [1600/20/30/256 (TR/TE/TM/acquisitions)].
The main resonances correspond to N-acetylaspartate (NAA, 2.0 ppm), glutamate/glutamine (Glx, 2.1-2.5 ppm), creatine/phosphocreatine (Cr, 3.02 ppm), choline-containing compounds (Cho, 3.2 ppm), and myo-Inositol (mIns, 3.55 ppm). Comparison of the spectra shows a decrease in Cho and mIns resonances with an increase in the glutamate/glutamine region in the cirrhotic patient. Reproduced with the permission of Springer Science and Business Media, from Cordoba et al 2002[28].
The choline resonance contains contributions from phosphocholine and glycerophosphorylcholine, which are cell membrane precursor and degradation products[86]. This observed reduction is likely to reflect alterations in phospholipid metabolism, membrane fluidity, or secondary changes in water content, because glycerophosphorylcholine is itself a cerebral osmolyte[87].
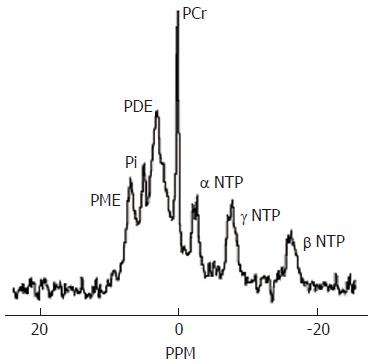
The typical cerebral 31P MR spectrum contains at least seven resonances, which can be assigned to phosphomonoesters (PME), inorganic phosphate (Pi), phosphodiesters (PDE), phosphocreatine, ATP, ATP and ATP (Figure 6)[88]. 31P MRS provides information on the status of energy phosphates and on phospholipid metabolism. Compared to 1H MRS, there is less agreement in the published cerebral 31P findings in patients with HE, due to the small populations studied and variation in the techniques that have been used. Early studies reported reductions in cerebral inorganic phosphate or phosphocreatine, which were interpreted as indicating defects in cerebral energy metabolism[89]. Our group has consistently found reductions in the PME/ATP and PDE/ATP ratios, which correlate with the reduction in choline using 1H MRS[83,84,90,91]. A recent study which used proton-decoupled 31P MRS, a technique which allows the components of the composite PDE peak to be resolved, showed a reduction in glycerophosphorylcholine, which is a major component of PDE and, as discussed above, may be reduced as a consequence of disturbances of cerebral osmoregulation[87].
Figure 6 Normal brain phosphorus-31 MR spectrum acquired at 1.
5T. PME = phosphomonoesters, Pi=inorganic phosphate, PDE = phosphodiesters, PCr = phosphocreatine, NTP = nucleotide triphosphate. The PME resonance provides information on cell membrane precursors and the PDE resonance on cell membrane degradation products.
FUTURE MRS DEVELOPMENTS
The current limitations of MRS include the limited resolution of metabolites which resonate within a narrow spectral range, such as glutamine and glutamate, and the inability to detect low concentration metabolites. Possible advances in the field include the use of two-dimensional spectroscopy, spectral editing and the use of higher magnetic field strength systems (for example at 3T).
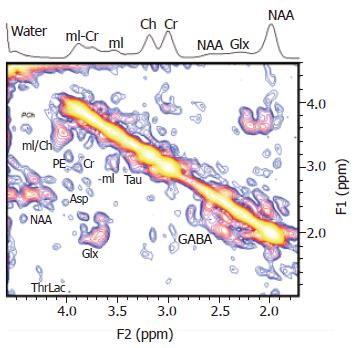
Of these, two-dimensional spectroscopy has already been used to investigate HE. Investigators have reported enhanced spectral resolution with the additional finding of an increased taurine resonance, which was well resolved [92] (Figure 7). However, currently this technique suffers from low signal-to-noise, and despite the use of a surface head coil, it is unable to probe the deeper grey matter structures, such as the basal ganglia.
Figure 7 A localized two-dimensional correlated spectroscopy (two-dimensional L-COSY) spectrum of a 40-year-old patient.
A projected one-dimensional spectrum is also shown on top of the figure. Peaks are presented in the magnitude mode. Reproduced with permission from John Wiley and Sons Inc, from Binesh N et al 2005[92].
Spectral editing involves only the acquisition of a desired spectrum of interest by suppressing the signal of other metabolites and then attempting to acquire only the signal of interest. This approach is of importance in HE in order to separate glutamate from glutamine, which overlap in conventional MR spectra. It should be noted that spectral editing is particularly sensitive to motion artefact and instrument instability. However, these methodologies have been used to highlight taurine, GABA and myo-inositol in vivo and suppress unwanted resonances[93-95]. Such sequence development promises much in the future for HE patients, but with respect to resolving glutamate from glutamine in vivo, another approach called averaged TE spectroscopy has been used to resolve glutamate from glutamine[96]. This has been used in multiple sclerosis at 3T, but not yet in HE patients[97].
With the advent of clinical magnet systems operating at higher magnetic fields, such as 3T, which is twice that used hitherto in published studies, the sensitivity of the technique may be enhanced further with improved signal-to-noise ratios. The possibility of performing multiple MR sequences in the same examination of the brain is now possible, because acquisition times are usually faster at these higher field strengths. It will thus be feasible to perform MRS studies on cerebral osmolyte levels in the same examination as acquiring data suitable for computer-enhanced visual detection of brain swelling and fMRI which should pinpoint cognitive and perceptive derangements. Since MR does not involve ionising radiation, serial studies will be possible to look at treatment effects in HE.
CONCLUSIONS
In vivo MRI and MRS provide a non-invasive way of studying brain chemistry and metabolism in patients with liver disease. These techniques have been central in the study of the underlying mechanisms of liver-related brain disease. MRS remains a research tool in the setting of liver disease. However, simple MRS sequences can be added to a standard MR examination to provide metabolic information that could help monitor response to therapy. MR studies in HE have provided important in vivo data, which have contributed to the current theories on astrocyte swelling[5]. This model accommodates many of the common precipitating factors for CHE, such as hyponatremia, benzodiazepine administration and infection (raised inflammatory cytokines), since these events also lead to astrocyte swelling in vitro[5]. Astrocyte swelling may therefore be a common pathway for multiple synergistic factors in the pathogenesis of HE complicating chronic liver disease. However, further MR studies are clearly required before these ascertations can be confirmed. A whole variety of new MR techniques now exist to study HE in more depth.
ACKNOWLEDGEMENTS
Studies performed at the Robert Steiner MRI Unit, MRC Clinical Sciences Centre, Hammersmith Hospital Campus, Imperial College London, are supported by the United Kingdom Medical Research Council (G99000178), the Royal College of Physicians of London, Philips Medical Systems (Cleveland, Ohio, USA) and the United Kingdom Department of Health. Dr Grover is currently supported by a grant from the Paddington Charitable Trust, St Mary’s, London, United Kingdom.