Published online Apr 21, 2006. doi: 10.3748/wjg.v12.i15.2450
Revised: November 2, 2005
Accepted: November 18, 2005
Published online: April 21, 2006
AIM: To investigate the effect of angiopoietin-1 (Ang-1) on biological behaviors in vitro and tumorigenesis and angiogenesis in vitro of human gastric cancer cells.
METHODS: Human full-length Ang-1 gene was cloned from human placental tissues by RT-PCR method. Recombinant human Ang-1 antisense eukaryotic expression vector was constructed by directional cloning, and transfected by lipofectin method into human gastric cancer line SGC7901 with high Ang-1 expression level. Inhibition efficiency was confirmed by semi- quantitive PCR and Western blot method. Cell growth curve and cell cycle were observed with MTT assays and flow cytometry, respectively. Nude mice tumorigenicity test was employed to compare in vitro tumorigenesis of cells with Ang-1 suppression. Microvessel density (MVD) of implanted tumor tissues was analyzed by immunohistochemistry for factor VIII staining.
RESULTS: Full-length Ang-1 gene was successfully cloned and stable transfectants were established, namely 7Ang1- for antisense,and 7901P for empty vector transfected. 7Ang1- cells showed down-regulated Ang-1 expression, while its in vitro proliferation and cell cycle distribution were not significantly changed. In contrast, xenograft of 7Ang1- cells in nude mice had lower volume and weight than those of 7901P after 30 days’ implantation (P < 0.01, 293.00 ± 95.54 mg vs. 624.00 ± 77.78 mg) accompanied with less vessel formation with MVD 6.00±1.73 compared to 7901P group 8.44±1.33 (P < 0.01).
CONCLUSION: Ang-1 may play an important role in tumorigenesis and angiogenesis of gastric cancer, and targeting its expression may be beneficial for the therapy of gastric cancer.
- Citation: Wang J, Wu KC, Zhang DX, Fan DM. Antisense angiopoietin-1 inhibits tumorigenesis and angiogenesis of gastric cancer. World J Gastroenterol 2006; 12(15): 2450-2454
- URL: https://www.wjgnet.com/1007-9327/full/v12/i15/2450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i15.2450
Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), two main members of angiopoietins family, are involved in both physiological and pathological angiogenesis processes. Ang-1 has been identified as a major activator of the tyrosine kinase receptor Tie2, leading to receptor autophosphorylation on binding. Ang-1 also stimulates endothelial cell migration in vitro[1,2]. Ang-2 is the naturally occurring antagonist to Ang-1 and inhibits Ang-1 mediated Tie2 phosphorylation; this effect leads to vessel destabilization, a necessary step in the initiation of angiogenesis[3,4].
Ang-1 and Ang-2 have been reported to be involved in several kinds of cancers, while their roles in gastric cancer progression and angiogenesis are still not fully understood. There have been several reports declaimed the effect of Ang-1 on tumorigenesis of human astrocytomas[5,6], breast cancer[7] and colorectal adenocarcinoma[8]. Previous report has identified that Ang-2 plays an important role in gastric cancer angiogenesis and progression[9,10], while little is known about the roles of Ang-1 in gastric cancer.
In the present study, we aimed to investigate the effect of Ang-1 on the biological behaviors and tumorigenesis and angiogenesis of human gastric cancer cells by modifying Ang-1 expression in SGC7901 gastric cancer cells by in vivo and in vitro examinations. Our results revealed that inhibition of Ang-1 expression would retard gastric cancer angiogenesis and progression.
Fresh placental tissue was obtained from the Department of Gynaecology and Obstetrics, Xijing Hospital, Xi’an, China, with informed consent from the patients. Human gastric cancer cell line SGC7901 with high Ang-1 expression was preserved in our institute and cultured in RPMI1640 supplemented with 100 mL/L bovine serum.
Ang-1 antisense eukaryotic expression vector was conducted by RT-PCR method and directional cloning. Total RNA of fresh human placental tissue was extracted with Trizol (Life Technologies, Carlsbad, USA) according to the manufacturer’s protocol. About 1 μg of total RNA was used for first strand cDNA synthesis according to the manufacturer’s instructions. The full-length human Ang-1 cDNA was cloned using primer pairs: 5’-gagggggaaagagtcaaacaaac-3’ and 5’-cttgaccgtgaatctggagcc-3’. PCR parameters were 94 °C for 1 min, annealing at 60 °C for 1 min, and 72 °C for 2 min for 30 cycles, and the product was verified by 8 g/L gel electrophoresis. Sequence of PCR product was verified by the ABI PRISM 377 DNA Sequencer (Sangon, Shanghai, China). After PCR, the 1.9-kb fragment was cloned into the pMD18-T vector (Takara, Dalian, China) followed by HindIII and BamHIdigestion and ligated into pcDNA3.1-V5-His C expression vector (Invitrogen, Carlsbad, CA) named as pcDNA3.1-Ang1-. The sequence was confirmed by sequencing analysis.
SGC7901 cells were plated and grown to 80% confluency without antibiotics. Stable transfections of pcDNA3.1-Ang1- and empty vector, pcDNA3.1-V5-His C, were performed with Lipofectamine 2 000 (Invitrogen, Carlsbad, CA) as directed by the manufacturer. Transfected cells were selected with 300 mg/L G418 after 48 h. Clones were picked and expanded for an additional 2 months. Efficiency of Ang-1 suppression was confirmed by RT-PCR and Western blot methods.
Semi-quantitative RT-PCR was employed to compare the gene expression in different transfectants. The number of optimal replications was determined based on the linear correlations between cycle numbers and PCR products. Gene expression was presented by the relative yield of the PCR product from target sequences to that from GAPDH gene as control. PCR primers and reaction parameters were chosen according to the literature [11] and listed as follows: Ang-1, 5’-ACTGTGCAGATGTATATCAAGC-3’ and 5’-GTGGAATCTGTCATACTGTGA A-3’; GAPDH, 5’ -TGGGTGTGAACCATGAGAAGTA-3’ and 5’-CGCTGTTGAAGTCAG AGGAGA-3’. The PCR parameters were 94°C for 1 min, annealing at 60°C for 1 min, and 72°C for 1 min for 32 cycles for Ang-1 and 94°C for 30 s, annealing at 52°C for 30 s, and 72°C for 45 s for 32 cycles for GAPDH.
Equal amounts of the extracted protein of cells were separated by 100 g/L SDS–PAGE; The protein bands were electro-transferred to nitrocellulose membrane. Expression of Ang-1 was analyzed using primary antibody (kindly provided by Micheal Hanner from Renegeron Pharmaceuticals, USA), followed by corresponding second antibody (Zhongshan, Beijing, China ), then visualized by enhanced chemiluminescence (ECL, Amersham–Pharmacia Biotech, Beijing, China). Relative protein levels were calculated to β-actin as standard.
Exponentially growing cells were harvested and suspended in 96-well flat-bottomed plates (200 µL /well) (Costar). After incubation for 1, 3, 5 and 7 d, respectively, 20 µL of MTT solution (5 g/L) (Sigma, St louis, MO) was added to each well and further incubated for 4 h. One hundred and fifty µL of dimethylsulfoxide was added before absorbance at 490 nm was measured with a microplate reader BP800 (BIOHIT).
To evaluate whether the inhibition of Ang-1 would exert any effect on the cell cycle distribution of SGC7901 cells, we performed cytofluorimetric analysis. Briefly, cells were fixed with 70% ice-cold ethanol and stored at 4°C overnight, washed with PBS, and stained with propidium iodide (PI) (50 mg/L) for 30 min. Cell cycle histograms were generated after analysis of PI-stained cells by fluorescence activated cell sorting (FACS) with a Becton-Dickinson FACScan.
To investigate whether or not Ang-1 suppression would alter the tumorigenicity of SGC7901 cells, nude mice bred in our animal facilities were used with body weight range from 18 to 22 kilograms. Four × 106 cells were injected subcutaneously into the right flank of nude mice. The experiment was performed on five mice in each group. The mean tumor volume was weekly measured and calculated according to the formula: a × (b) 2× 0.5 (a = largest diameter, b = perpendicular diameter). All mice were sacrificed after 30 d and the subcutaneous tumor graft were surgically excised from the mice, fixed with formalin and paraffin-embedded 4-μm sections were prepared for immunohistochemical analysis.
Factor VIII staining was used to identify the microvessels in the tumor tissues by immunohistochemical method as usual. Briefly, after paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated in alcohol, sections were incubated in 3 mL/L H2O2 to block endogeneous peroxidase activity. Each slide was incubated with normal goat serum for 20 min at room temperature, then rabbit anti-factor VIII related antigen antibody (Dako, MedBio Ltd, New Zealand) diluted at 1 : 100 was applied on sections and incubated overnight at 4°C. After incubation with biotinylated goat anti-rabbit IgG (dilution 1:200) for 30 min at 37°C, each slide was rinsed in phosphate-buffered saline and was incubated in the avidin-biotin peroxidase complex for 30 min at 37°C. The peroxidase was visualized with 3-3’-diamino- benzidinetetrahydrochloride (DAB) solution and then counterstained with hematoxylin.
MVD was assessed according to the international consensus report[12]. Immuno stained slides were scanned at × 100 magnification to identify the areas with the highest number of vessels (so called “hot spot”). Counts were performed on five fields in the hot spot by two independent pathologists at × 200 magnification and the mean was taken and analyzed.
Statistical analysis was performed using SPSS software (version 10.0, SPSS Inc, Chicago). The ANOVA test was used to compare the differences between groups for MVD, tumor weight and volume. Differences were considered statistically significant at P <0.05.
Full length Ang-1 gene was amplified from placental tissues and cloned into pMD 18-T vector, then cut by by HindIII and BamHIdigestion which would ensure inverse directional ligation into pcDNA3.1-V5-His C expression vector. The combined antisense expression vector named as pcDNA3.1-Ang1- was verified as expected by sequencing.
Stable transfectants of 7901-derived cells were obtained by Lipofectamine 2000 transfection, named 7Ang1- for pcDNA3.1-Ang1- and 7901P for empty vector- transfected cells. We could clearly find that moderate targeted-inhibition effect was detected in 7Ang1- cells at both mRNA (Figure 1A) and protein (Figure 1B) levels, in contrast, control experiment with the empty vector alone showed a negligible effect on Ang-1 expression (Figure 1).
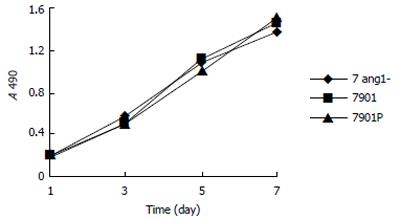
To determine if interference of Ang-1 expression has an effect on the proliferation and cell cycle distribution of SGC7901 cells, MTT assay and FACS method were utilized, respectively. According to Figure 2, the proliferation of 7Ang1- cells was not significantly changed compared to that of 7901P cells. In addition, cell cycle distribution of Ang1- and 7901P were 71.9% and 59.2% for G1 phase, 15.3% and 12.5% for G2 phase, 25.5% and 15.6% for S phase, 0.328 and 0.281 for the proliferation index, respectively. Taken together, it suggests that interfering Ang-1 has no direct effect on in vitro proliferation and cell cycle of gastric cancer cells.
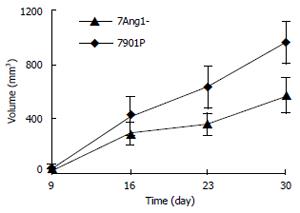
Xenograft model was employed to compare the tumorigenicity of SGC7901 cells before and after Ang-1 inhibition. Subcutaneous tumor node of different groups became palpable almost simultaneously after 7d transplantation. Tumor from 7Ang1- cells appeared to grow slower than those from 7901P cells after 16 d (Figure 3). Finally, tumor grafts were collected and weighed after 30 d, and tumor tissues derived from 7Ang1- cells showed significantly decreased weight compared to those from 7901P cells with mean tumor graft weight (mg) being 293.0 ± 95.5 for 7Ang1- and 624.0 ± 77.8 for 7901P cells (P < 0.01).
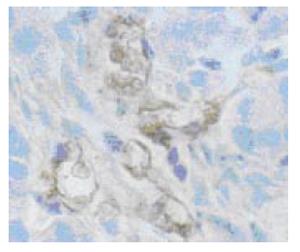
To further elucidate whether reduced angiogenesis account for the suppressed in vivo growth of 7Ang1- cells, MVD was assessed by immunohistochemistry. As shown in Figure 4, microvessels could easily be observed by factor VIII staining. Statistics analysis showed a significantly less MVD was present in 7Ang1- group 6.0 ± 1.7 compared to 8.4 ± 1.3 in 7901P group (P < 0.05). This result indicated that suppressed in vivo tumorigenicity of 7Ang1- cells might be mediated through reduced angiogenesis.
Gastric cancer remains a common malignancy in many countries of the world, especially in Asia, and is still among the most important causes of cancer-related death worldwide[13]. Its conventional treatment includes surgery, radiation and chemotherapy. Nowadays, increasing evidence has shown that angiogenesis is essential for the growth of solid tumor and tumor angiogenesis research has become one of the most active fields for anticancer therapies.
Several studies suggested that VEGF receptor pathway and Tie2 pathway are independent and essential mediators of angiogenesis and both play important roles in the tumor angiogenesis[14,15]. Tie2 is a novel endothelial cell-specific molecule involved in both physiological and pathological processes. Tie2 is required for normal vascular development perhaps via the regulation of vascular remodeling and endothelial cell interactions with supporting pericytes and smooth muscle cells[3]. Previous report found that inhibition of Tie2 using a kinase-deficient Tie2 construct or an adenoviral vector delivering a recombinant single-chain antibody fragment into body would inhibit the growth of human tumor xenografts[16,17].
Four ligands for the Tie2 receptor have been identified so far, named Ang-1 to -4. Among them, Ang-1 and -2 were mainly associated with tumor angiogenesis[18]. The findings from functional study of Ang-2 in several types of tumor showed that Ang-2 could stimulate tumor angiogenesis, thus promoting tumor progress[9,19,20], while those from Ang-1 showed great heterogeneity. For example, Zadeh et al[6] found that Ang-1 increased the vascular growth of both subcutaneous and intracranial xenografts of astrocytoma; also, Ang-1 promoted tumor angiogenesis and tumor vessel plasticity of human cervical cancer in mice[21]. In contrast, others have reported that Ang-1 could inhibit angiogenesis and growth of hepatic and colon cancer[22] and suppress breast cancer xenograft angiogenesis by blocking tumor neovasculature[7]. Taken together, it suggests that the role of Ang-1 in tumor progression may be context-dependent.
Our previous study found that Ang-1 was highly expressed in SGC7901 cells[23], while its expression in 7Ang1- cells was effectively reduced using antisense method in the present study. Further study revealed that the biological behaviors including proliferation and cell cycle of 7Ang1- cells were not significantly changed, so it seemed that interfering Ang-1 could not alter in vitro growth of SGC7901 cells directly; this is similar to other studies[9] in which growth of the gastric cancer cells was not affected by transfection with Ang-2.
Tumorigenicity assay showed that tumor xenograft of 7Ang1- group grew slower as their volume and weight were smaller than those from control group. MVD assessment showed significantly decreased angiogenesis in tumor from 7Ang1- group, indicating inadequate blood supply might account for suppressed in vivo growth. To our best knowledge, this is the first direct evidence for the role of Ang-1 in gastric cancer angiogenesis. However, in initial stage of tumor progression, two groups did not show significant difference, and until 16 d after transplantation the suppressive effect appeared dramatic when tumor grew larger. This result is consistent with our previous study in which suppressing the expression of VEGF in SGC7901 cells did not exert any significant effect on its proliferation but restricted its in vivo growth in nude mice[24]. Collectively, it infers that suppressing either VEGF or Ang-1 cannot prohibit the onset of tumor, but may slow in vivo growth of tumor cells by inhibiting angiogenesis. It was reported that Ang-2 was implicated in angiogenesis and progression of gastric carcinoma via induction of proteases such as MMP-1 and -9[9]. Combining result from our present and previous studies that significant expression of Ang-1 and Ang-2 was found in human gastric cancer tissues and cell lines[23], it infers that both Ang-1 and -2 participate in the angiogenesis and progression of gastric cancer. Previous studies indicated that Ang-1 induced the secretion of MMP-2 and small amounts of proMMP-3 and proMMP-9 in endothelial cells[25], whether or not this effect is involved in the mechanism of Ang-1 contribution to gastric cancer and progression is still unclear, however it still could be concluded from our present study that stimulating angiogenesis by Ang-1, in part at least, could promote tumor progress.
In summary, Ang-1 may contribute to the progression of gastric cancer and inhibiting its expression in gastric cancer cells could suppress in vivo tumorigenicicy. It could also be a useful therapeutic target to prevent gastric cancer or inhibit its malignant progression. Tumor angiogenesis is a multi-factor associated process, and recent study showed that simultaneous phenotypic knockout of VEGF-R2 and Tie2 with an intradiabody could effectively reduce tumor growth and angiogenesis in vivo[26]. It suggests that combination of targeting main angiogenesis modulators including VEGF, Ang-1 and Ang-2 and their corresponding receptors can be beneficial for tumor therapeutics.
| 1. | Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2170] [Cited by in RCA: 2057] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 2. | Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1417] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 3. | Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2548] [Cited by in RCA: 2555] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 4. | Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1620] [Cited by in RCA: 1539] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 5. | Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Zadeh G, Koushan K, Pillo L, Shannon P, Guha A. Role of Ang1 and its interaction with VEGF-A in astrocytomas. J Neuropathol Exp Neurol. 2004;63:978-989. [PubMed] |
| 7. | Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY. Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer. 2002;86:645-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Nakayama T, Hatachi G, Wen CY, Yoshizaki A, Yamazumi K, Niino D, Sekine I. Expression and significance of Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in colorectal adenocarcinoma: Immunohistochemical analysis and correlation with clinicopathological factors. World J Gastroenterol. 2005;11:964-969. [PubMed] |
| 9. | Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145-2153. [PubMed] |
| 10. | Sun XD, Liu XE, Wu JM, Cai XJ, Mou YP, Li JD. Expression and significance of angiopoietin-2 in gastric cancer. World J Gastroenterol. 2004;10:1382-1385. [PubMed] |
| 11. | Wong MP, Chan SY, Fu KH, Leung SY, Cheung N, Yuen ST, Chung LP. The angiopoietins, tie2 and vascular endothelial growth factor are differentially expressed in the transformation of normal lung to non-small cell lung carcinomas. Lung Cancer. 2000;29:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 504] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 14. | Siemeister G, Schirner M, Weindel K, Reusch P, Menrad A, Marmé D, Martiny-Baron G. Two independent mechanisms essential for tumor angiogenesis: inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res. 1999;59:3185-3191. [PubMed] |
| 15. | Stratmann A, Acker T, Burger AM, Amann K, Risau W, Plate KH. Differential inhibition of tumor angiogenesis by tie2 and vascular endothelial growth factor receptor-2 dominant-negative receptor mutants. Int J Cancer. 2001;91:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Zadeh G, Qian B, Okhowat A, Sabha N, Kontos CD, Guha A. Targeting the Tie2/Tek receptor in astrocytomas. Am J Pathol. 2004;164:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Popkov M, Jendreyko N, McGavern DB, Rader C, Barbas CF 3rd. Targeting tumor angiogenesis with adenovirus-delivered anti-Tie-2 intrabody. Cancer Res. 2005;65:972-981. [PubMed] |
| 18. | Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255-1259. [PubMed] |
| 20. | Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci U S A. 2003;100:8904-8909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Shim WS, Teh M, Bapna A, Kim I, Koh GY, Mack PO, Ge R. Angiopoietin 1 promotes tumor angiogenesis and tumor vessel plasticity of human cervical cancer in mice. Exp Cell Res. 2002;279:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Stoeltzing O, Ahmad SA, Liu W, McCarty MF, Wey JS, Parikh AA, Fan F, Reinmuth N, Kawaguchi M, Bucana CD. Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 2003;63:3370-3377. [PubMed] |
| 23. | Wang J, Wu K, Zhang D, Tang H, Xie H, Hong L, Pan Y, Lan M, Hu S, Ning X. Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochem Biophys Res Commun. 2005;337:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Liu DH, Zhang XY, Huang YX, Fan DM. VEGF165 antisense gene and biological characteristics of human gastric cancer cells. Disi Junyi Daxue Xuebao. 2001;22:821-824. |
| 25. | Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Jendreyko N, Popkov M, Rader C, Barbas CF. Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:8293-8298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
S- Editor Pan BR L- Editor Zhang JZ and Zhu LH E- Editor Ma WH