Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2139
Revised: July 11, 2005
Accepted: July 20, 2005
Published online: April 7, 2006
Previous reports of a solitary metastatic hepatocellular carcinoma have been rare. Because this tumor has a different treatment modality and prognosis, an accurate differential diagnosis is essential. Here we report a rare case of a solitary chest wall metastasis from unknown primary site of hepatocellular carcinoma. It involves a 51-year-old man who was admitted to our hospital because of a palpable left upper chest wall mass. The mass was resected and pathologic examination confirmed a diagnosis of metastatic hepatocellular carcinoma. Despite our investigation, no evidence was found that indicated the primary origin of the hepatocellular carcinoma. Four months later, the patient was admitted again because of spinal cord compression at the third and fourth thoracic vertebrae. Emergent decompressive laminectomy was performed and microscopic features revealed the same pathology as the initial chest wall mass resected 4 months earlier. After one year, a follow-up abdominal computed tomography (CT) still revealed no evidence of primary hepatocellular carcinoma.
- Citation: Hyun YS, Choi HS, Bae JH, Jun DW, Lee HL, Lee OY, Yoon BC, Lee MH, Lee DH, Kee CS, Kang JH, Park MH. Chest wall metastasis from unknown primary site of hepatocellular carcinoma. World J Gastroenterol 2006; 12(13): 2139-2142
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2139
The most common causes of a chest wall mass in adults are infection or tumor,[1] and the majority of the malignant masses are hematological in origin. Malignant chest wall masses arising from a primary focus in the lung (metastatic lung cancer) or the liver (metastatic hepatocellular carcinoma) are rare.[2] Primary hepatocellular carcinoma often metastasizes out of the liver. Metastasis typically occurs in patients with advanced stages of the disease, and the most common site is the lungs, followed by the abdominal wall, lymph nodes, bone, adrenal gland, pancreas, kidney and spleen.[3,4] Katyal et al [5] categorized the intrahepatic stage of the tumor according to TNM staging, and reported that extrahepatic metastasis of small hepatocellular carcinomas (confined to stage one) was rare. References indicates that hepatocellular carcinomas found outside the liver without a primary focus are usually due to carcinogenesis of ectopic liver. Cases of hepatoid adenocarcinoma have previously been reported, but a solitary extrahepatic hepatocellular carcinoma that has metastasized to the chest wall has rarely been reported. Because these types of tumors have different treatment modalities and prognoses, an accurate differential diagnosis is essential. Here, the authors have diagnosed a solitary metastatic hepatocellular carcinoma of the chest wall, with an unknown primary focus, and have been closely following the patient for one year since the diagnosis.
A 51-year-old male visited the hospital because of a left upper chest wall mass that developed during the prior 8 months. Ten years ago he was diagnosed with chronic viral hepatitis B but was lost to follow-up in the intervening period. The patient had not received any treatment since the mass was first discovered, and the mass progressively grew in size until the patient sought treatment at the hospital. The patient was a heavy drinker and smoker, with a drinking history of two bottles of beer per week for 30 years, and a smoking history of 15 pack years. There was no significant family history. The patient's initial vital signs were stable and he appeared relatively well, and was alert and responsive. On physical examination, a hard, palpable mass about 10 cm × 12 cm in size was noted on the left upper anterior chest wall. Palmar erythema and spider angioma on the anterior chest wall were noted, but there were no other specific complaints. Initial laboratory findings were as follows: WBC 10 000/mm3, hemoglobin 134 g/L, platelets 216 000/mm3, serum protein 56 g/L, albumin 34 g/L, total cholesterol 214 mg/dL, serum glucose 113 mg/dL, total bilirubin 0.9 mg/dL, AST 38 U/L, ALT 52 U/L, alkaline phosphatase 104 U/L, and prothrombin time 135%. Patient's viral markers were as follows: HBsAg(+), HBcAb(+), HBeAg(-), HBeAb(+) and HBV DNA < 2.5pg. Serum alpha-fetoprotein was 308.3 ng/mL.
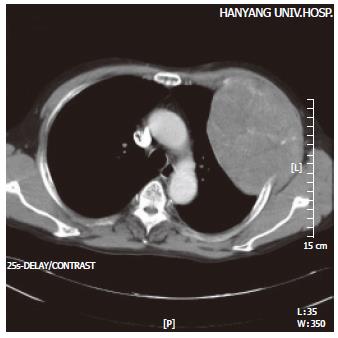
The density of the soft tissue protruding inwards towards the lung was noted, with accompanying evidence of rib destruction along the anterolateral aspect of the third left rib on the chest film. A 9.5 cm× 12.7 cm sized heterogeneous tumor with a mottled calcification was noted on the anterolateral aspect of the left chest wall on the CT, but there was no evidence of metastasis within the lung parenchyma or the mediastinum (Figure 1). An additional study of abdominal ultrasonography and whole body bone scan revealed no evidence of an intrahepatic mass, abdominal lymphadenopathy, or bone metastasis. A primary bone tumor on the left upper chest wall was suggested, and radical resection of the tumor around the rib and soft tissue was performed.
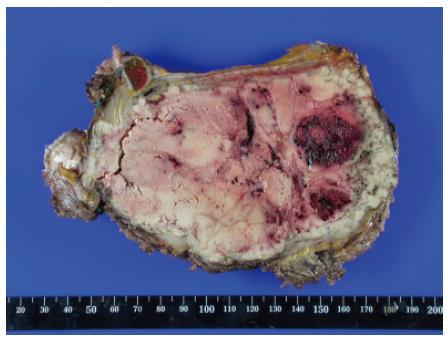
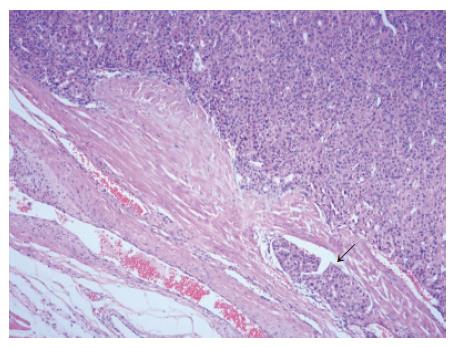
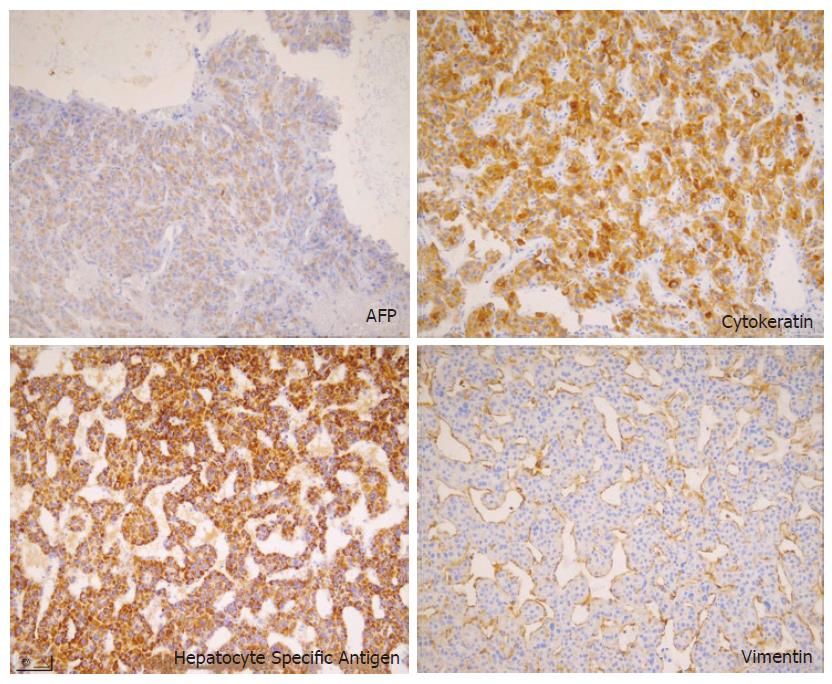
The resected tumor was well-encapsulated with dimensions 14 cm× 10 cm× 7.5 cm and weighed 650 mg including the excised rib tissue. The cut surface of the tumor was whitish-brown with accompanying hemorrhage and necrosis, and was solid and hard but tended to crumble easily (Figure 2). Microscopic examination revealed large polygonal cancer cells separated by thin vessels resulting in a pole-like arrangement, and multiple cells undergoing mitosis were seen. Cancer cell emboli were seen inside the vessels surrounding cancer cells, and there was no evidence of normal liver tissue within the microscopic field (Figure 3). Immunohistochemical staining showed positive results for alpha-fetoprotein, hepatocyte specific antigen and cytokeratin, and showed negative results for vimentin and smooth muscle actin (Figure 4).
Abdominal computerized tomography (CT) and abdominal magnetic resonance imaging (MRI) were performed in search of the primary focus of the hepatocellular carcinoma. The surface of the liver was slightly irregular which was suspicious of liver cirrhosis, but there was no evidence of hepatocellular carcinoma, and no lipiodol uptake nor tumor vessels were seen in the hepatic arteriography. Mild lobular necrosis with mild portal inflammation and macronodular cirrhosis on the portal area were noted upon liver biopsy, but there was no evidence of a tumor. Upon esophagogastroduodenoscopy, antral gastritis was present but there were no gastric or esophageal varices, and there was no significant sigmoidoscopic finding. Post-operative alpha-fetoprotein level decreased from 308.3 ng/mL to 42.4 ng/mL. Follow-up abdominal CT was performed three weeks after the first hepatic arteriography, and like before, no lipiodol uptake was seen. There were no operation-related complications, and the patient was discharged to be regularly monitored as an outpatient.
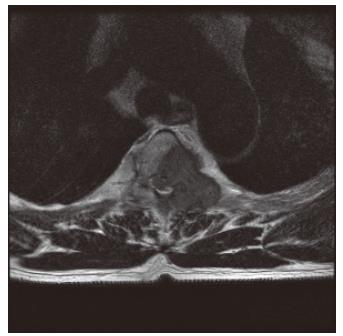
Four months after discharge, the patient visited the emergency room due to back pain radiating to both lower extremities. On physical examination, there was evidence of motor weakness in both lower extremities, and the patient complained of paresthesia and hyperesthesia in the dermatome below the forth thoracic vertebra. Immediate thoracic MRI revealed spinal cord compression due to a metastatic lesion to the third and forth thoracic vertebrae, with spinal deviation to the right of the posterolateral position (Figure 5). Emergent decompressive laminectomy was performed. Further evaluation with a whole body bone scan revealed a newly developed increased uptake at the left side of the third to fifth thoracic vertebrae, while abdominal ultrasonography showed no significant change from the initial diagnosis of metastatic hepatocellular carcinoma in the chest wall. Histological examination of the metastatic vertebral lesions revealed metastatic hepatocellular carcinoma, which was the same finding observed during microscopic examination of the chest wall mass. Afterwards the patient received radiotherapy and adriamycin-based chemotherapy. One year later, no lesion suggesting primary hepatocellular carcinoma has been found in the follow-up abdominal CT scan.
An extrahepatic hepatocellular carcinoma without a primary intrahepatic hepatocellular carcinoma can be explained in two different ways. The first is ectopic liver carcinogenesis and the second is a hepatoid adenocarcinoma.[6-8]
Ectopic liver is a rare developmental abnormality that can originate in the gall bladder, hepatic ligament, omentum, posterior peritoneal cavity, or the thorax.[8-11] Because ectopic liver does not have perfect functional architecture as does a normal liver, it is more likely to develop hepatocellular carcinoma, if carcinogenic factors such as viral hepatitis infection or liver cirrhosis are involved.[6]
Characteristically, the pathologic examination of hepatocellular carcinoma arising from ectopic liver reveals normal liver tissue, including portal triads. It may be connected to the liver by a fibrous stalk, which is composed of the portal vein, hepatic artery, or bile duct. If no evidence of primary cancer of the liver is present after a long term follow-up with various liver imaging studies, a malignancy originating from ectopic liver should be suspected .[7] In Japan, 25 cases of hepatocellular carcinoma of ectopic liver were described. This can be treated with complete resection of the tumor, which yields a good prognosis.
Hepatoid adenocarcinoma is a variant form of adenocarcinoma, characterized by vast hepatic differentiation. It produces alpha-fetoprotein, while having the same function and form as hepatocellular carcinoma, but originates from the endoderm and mucosa of urogenital organs and therefore can manifest itself in the gastrointestinal tract, ovaries, pancreas, lungs, kidneys, uterus or bladder. It contains a characteristic moderately to well-differentiated tubular or papillary shaped adenocarcinoma[8].
The present case differs from the two aforementioned examples because it was discovered as a solitary metastatic hepatocellular carcinoma of the chest wall, with an unknown primary focus. First, it was composed solely of hepatocellular carcinoma, and microscopic examination revealed no evidence of adenocarcinoma nor normal liver tissue including portal triads. This finding was entirely different from that expected in the carcinogenic sequence of ectopic liver or hepatoid adenocarcinoma. Second, cancer cell emboli were found within the vessels near the cancer cells, which is a characteristic microscopic finding of metastatic cancer. Thirdly, there was no evidence of an intrahepatic tumor in the liver imaging studies, and this result was again seen during the follow-up abdominal CT one year later. Fourthly, four months after resection of the chest wall tumor, metastatic hepatocellular carcinoma was found in the thoracic vertebrae. The thoracic vertebrae are a region of intense bone marrow replication, and are among the most important metastatic sites for hepatocellular carcinoma[12].
In summary, when hepatocellular carcinoma is found outside the liver without a primary focus within the liver, a tissue biopsy must be obtained. Tissue biopsy must confirm the presence of normal liver tissue, including portal triads and cancer emboli within vessels near the tumor, for an accurate differential diagnosis. Chest CT, abdominal ultrasonography, and whole body bone scans must be used in order to confirm the presence of metastasis to other organs. To discern between hepatocellular carcinoma of ectopic liver and solitary metastatic hepatocellular carcinoma without a primary focus in the liver, a follow-up study is essential after the operation in order to find the primary site of origin.
Although the patient had no evidence of an intrahepatic tumor, he was a hepatitis B carrier with liver cirrhosis which suggested a latent primary hepatocellular carcinoma metastasizing to the chest wall that spontaneously regressed. In cases like this, in which extrahepatic hepatocellular carcinoma exists without evidence of tumor within the liver, one has to consider the possibility of a tumor originating from ectopic liver tissue, hepatoid adenocarcinoma, or, although rare, metastatic hepatocellular carcinoma without an intrahepatic mass.
| 1. | Simpson L, Hanson M, Sheagren JN, Mallow J, Popalla S. An anterior chest wall mass. Am J Med. 2003;115:743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Toussirot E, Gallinet E, Augé B, Voillat L, Wendling D. Anterior chest wall malignancies. A review of ten cases. Rev Rhum Engl Ed. 1998;65:397-405. [PubMed] |
| 3. | Raoul JL, Le Simple T, Le Prisé E, Meunier B, Ben Hassel M, Bretagne JF. Bone metastasis revealing hepatocellular carcinoma: a report of three cases with a long clinical course. Am J Gastroenterol. 1995;90:1162-1164. [PubMed] |
| 4. | Doval DC, Rao CR, Acharya R, Reddy BK, Bapsy PP. Hepatocellular carcinoma metastatic to bones (Case report with review of literature). Indian J Cancer. 1995;32:31-35. [PubMed] |
| 5. | Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. [PubMed] |
| 6. | Arakawa M, Kimura Y, Sakata K, Kubo Y, Fukushima T, Okuda K. Propensity of ectopic liver to hepatocarcinogenesis: case reports and a review of the literature. Hepatology. 1999;29:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Asselah T, Condat B, Cazals-Hatem D, Hassani Z, Bernuau J, Groussard O, Mussot S, Lesèche G, Marcellin P, Erlinger S. Ectopic hepatocellular carcinoma arising in the left chest wall: a long-term follow-up. Eur J Gastroenterol Hepatol. 2001;13:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kishimoto T, Nagai Y, Kato K, Ozaki D, Ishikura H. Hepatoid adenocarcinoma: a new clinicopathological entity and the hypotheses on carcinogenesis. Med Electron Microsc. 2000;33:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Watanabe M, Matsura T, Takatori Y, Ueki K, Kobatake T, Hidaka M, Hirakawa H, Fukukmoto S, Shimada Y. Five cases of ectopic liver and a case of accessory lobe of the liver. Endoscopy. 1989;21:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Asada J, Onji S, Yamashita Y, Okada S, Morino M, Kanaoka M. Ectopic liver observed by peritoneoscopy: report of a case. Gastroenterol Endosc. 1982;24:309-312. |
| 11. | Clearfield HR. Embryology, malformation, and malposition of the liver. Bockus Gastroenterology. 4th ed. Philadelphia: Saunders; 1985; 2659-2665. |
| 12. | Cho SB, Lee SJ, Yoon KW, Joo YE, Kim HS, Choi SK. A case of metastatic small hepatocellular carcinoma to cranial bone with left eyelid ptosis. Korean J Hepatol. 2001;7:320-324. |
S- Editor Wang J L- Editor Pravda J E- Editor Zhang Y