Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2115
Revised: August 16, 2005
Accepted: August 26, 2005
Published online: April 7, 2006
AIM: To assess the lifetime cumulative incidence of portal venous thrombosis (PVT) in the general population.
METHODS: Between 1970 and 1982, 23 796 autopsies, representing 84% of all in-hospital deaths in the Malmö city population, were performed, using a standardised protocol including examination of the portal vein. PVT patients were characterised and the PVT prevalence at autopsy, an expression of life-time cumulative incidence, assessed in high-risk disease categories and expressed in terms of odds ratios and 95% CI.
RESULTS: The population prevalence of PVT was 1.0%. Of the 254 patients with PVT 28% had cirrhosis, 23% primary and 44% secondary hepatobiliary malignancy, 10% major abdominal infectious or inflammatory disease and 3% had a myeloproliferative disorder. Patients with both cirrhosis and hepatic carcinoma had the highest PVT risk, OR 17.1 (95% CI 11.1 - 26.4). In 14% no cause was found; only a minority of them had developed portal-hypertension-related complications.
CONCLUSION: In this population-based study, PVT was found to be more common than indicated by previous clinical series. The markedly excess risk in cirrhosis and hepatic carcinoma should warrant an increased awareness in these patients for whom prospective studies of directed intervention might be considered.
- Citation: Ögren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: Prevalence, patient characteristics and lifetime risk: A population study based on 23 796 consecutive autopsies. World J Gastroenterol 2006; 12(13): 2115-2119
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2115
Extrahepatic portal vein thrombosis (PVT) may be caused by a variety of conditions including cirrhosis, cancer and abdominal infectious and inflammatory processes. In the wake of thrombus organisation and vessel involution follows development of tortuous collaterals, so called cavernous transformation[1]. Symptom development is often insidious and related to the progression of portal hypertension. The concept of PVT as a rare disease is mainly based on clinical series and case reports[2-7]. Estimates of frequency and of distribution of etiological fractions vary widely between studies[2,4,5,6,8,9,10,11]. To some extent this may be explained by lack of precision in these small series, but a contribution of case selection and ascertainment bias should also be considered.
For several decades, the city of Malmö in southern Sweden has been a centre for epidemiological research, including clinical and autopsy-based studies of cardiovascular diseases[12,13,14,15]. Between 1970 and 1982, close to 24 000 autopsies, comprising 84% of all in-hospital deaths in the city, were performed. All procedures followed a standardised protocol including examination of the portal vein and its major tributaries. In consequence with the course in PVT, the prevalence at autopsy can be regarded as a proxy for the cumulative incidence during lifetime to develop PVT. The size of the cohort together with the high autopsy rate thus provides a unique opportunity to yield a robust estimate of risk in the general population. Further aims have been to evaluate likely causes to PVT and clinical characteristics related to portal hypertension, and to estimate the absolute risk of PVT in high-risk disease categories as characterised by autopsy findings.
Malmö is a city with a single referral centre for post-mortem examinations, the Department of Pathology at Malmö University Hospital. Between January 1, 1970 and December 31, 1982, when the city population declined from 264 000 to 230 000 inhabitants, 35 784 deaths occurred in the Malmö population. Among the 28 196 deaths occurring among in-patients at the three hospitals, which served the Malmö population during this period, a total of 23 796 clinical autopsies were performed, corresponding to an autopsy rate of 84%.
All autopsies were performed using a standardised protocol and carried out or supervised by senior pathologists. All findings were classified and coded according to the Standardised Nomenclature of Pathology (SNOP), as defined by the College of American Pathologists in 1965. The death certificates were issued by the pathologist. Based on the clinical picture and on autopsy findings, an underlying cause of death and up to six contributing causes were determined and classified using the ICD-8 code.
All cases with SNOP code 48-80-3700/3703, denoting portal vein thrombosis (PVT), were identified and validated individually. The following disease categories in the literature regarded as major causes of PVT were identified[5]:
-cirrhosis, where classification was based on SNOP codes 4850 - 4857 and/or cirrhosis as underlying or contributing death cause (ICD 8 code 571.00 - 571.99). PVT patients with cirrhosis were further classified according to presence or absence of primary hepatic cancer (hepatocellular carcinoma or intrahepatic cholangiocarcinoma);
-primary hepatobiliary cancer, comprising primary hepatic cancer (with or without cirrhosis), extrahepatic cholangiocarcinoma and gall bladder carcinoma;
-secondary malignancy of the hepatobiliary region (metastatic malignancy to the liver, biliary tract, gall bladder, duodenum or pancreas), further classified according to primary tumour location as expressed by SNOP code;
-myeloproliferative disorders: non-specific myeloproliferative disorder (SNOP code 7690); agnogenic myeloid metaplasia (7691); myelofibrosis (7692); histiocytic medullary reticulosis (7698); megablastic erythropoesis (7706); neutrophilic (7711) and eosinophilic (7713) leukocytosis; leukaemoid reaction (7714); erythrocytosis (7715); reticulocytosis (7716); lymphocytosis (7718); thrombocytosis (7719); erythrophagocytosis (7763); erythroid (7771), granulocytic (7772), reticular cell (7777) and megakaryocytic (7778) hyperplasia; polycythaemia vera rubra (7779); and chronic myeloic leukaemia (9867);
-major abdominal infections and inflammations, including liver abscess, abdominal abscess, purulent peritonitis, acute necrotising pancreatitis and ulcerative colitis.
In consequence with the possibility PVT being the result of more than one factor, a patient might be included in more than one of these categories. PVT patients in whom none of the above causes could be established were identified and analysed separately.
PVT prevalence and risk, expressed as odds ratio, were assessed in relation to the following disease categories: cirrhosis (with and without primary hepatic cancer), primary hepatobiliary cancer (with and without cirrhosis), secondary hepatic malignancy (secondary malignancy of the hepatobiliary region not being readily definable in terms of SNOP codes) and myeloproliferative disorders. Presence of secondary hepatic malignancy was based on SNOP code 56-8006. Among these, patients with metastatic disease from pancreatic, gastric and colorectal carcinoma were further identified and similarly analysed. For the other disease categories, patients were classified using the definitions stated in the previous section.
In patients with PVT, the prevalence at autopsy of the following complications typically related to portal hypertension was ascertained and analysed in relation to disease categories: ascites, oesophageal varices and gastrointestinal bleeding.
Distributions of age at death were expressed in terms of means and variance and groups defined by presence or absence of potential causal factor compared with one-way analysis of variance (ANOVA). Proportions were compared using two-sided Fisher’s exact test for univariate analyses. The PVT risks in relation to respective disease category were also expressed in terms of odds ratios with computation of 95% confidence intervals.
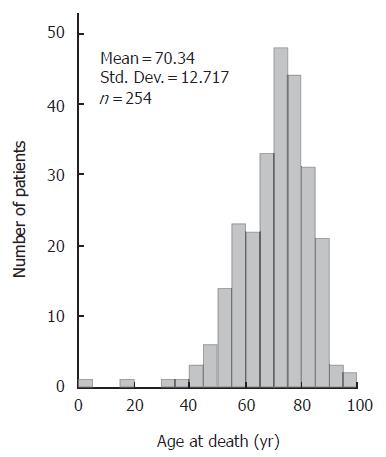
PVT was found at autopsy in 254 (1.1 %) of the 23 796 patients; in 125 (1.0 %) out of 12 157 men and 129 (1.1 %) of the 11 639 women (P = 0.57). The age distribution is depicted in Figure 1. With the exception of one patient, who was less than 1 year old, the age ranged between 17 and 96 years. Mean (95 % CI) age was 68 (66-70) years in men and 71 (69 - 74) years in women. Median age at death was 72 years; 70 years in men and 74 years in women.
Seventy-two (28 %) of the PVT patients had cirrhosis, and one third of those had also primary hepatic cancer (Table 1). In all 59 PVT patients (23 %) had primary hepatobiliary cancer and 111 (44 %) had secondary malignancy of the hepatobiliary region. Major abdominal infectious or inflammatory disease was present in 25 (10 %), 7 (3 %) had myeloproliferative disorders, whereas in 36 (14 %) of the patients with PVT, none of the above causal factors could be identified. The age distribution in these categories was similar (Table 1). Sixty-one per cent of the PVT patients with cirrhosis were men (P = 0.018), otherwise there were no gender differences.
| Patient category | n (%) ofPVT patients | n (%) withinrisk category | Age (yr)Median | Mean (95% CI) | Femalegender (%) |
| Cirrhosis 1 | 72 (28) | 70 | 68.5 (65.8 - 71.3) | 28 (39) | |
| with primary hepatic cancer | 26 (36) | ||||
| without primary hepatic cancer | 46 (64) | ||||
| Primary hepatobiliary cancer 1 | 59 (23) | 72 | 72.1 (69.4 - 74.8) | 26 (44) | |
| hepatic carcinoma 2 | 38 (64) | ||||
| extrahepatic biliary/gall bladder carcinoma | 21 (36) | ||||
| Secondary malignancy of the hepatobiliary region 1 | 111 (44) | 70 | 69.0 (66.7 - 71.2) | 59 (52) | |
| from pancreatic carcinoma | 47 (42) | ||||
| gastric carcinoma | 20 (18) | ||||
| colorectal carcinoma | 11 (10) | ||||
| lung cancer | 7 (6) | ||||
| malignant lymphoma | 5 (4) | ||||
| mammary adenocarcinoma | 4 (4) | ||||
| other primary cancer 3 | 17 (15)1 | ||||
| Myeloproliferative disorders 1 | 7 (3) | 72 | 65.4 (44.2 - 86.6) | 5 (71) | |
| Major abdominal infection / inflammation 14 | 25 (10) | 73 | 71.9 (66.5 - 77.3) | 15 (60) | |
| No cause identified | 36 (14) | 73 | 70.8 (65.9 - 75.7) | 21 (57) | |
| All patients with PVT | 254 (100) | 72 | 69.9 (68.3 - 71.4) | 129 (51) |
Cirrhosis was present in 5 % (1193 / 23796) of the cohort and related to a 7.9 times increased odds for PVT (95 % CI: 6.0-10.5) (Table 2). Patients who also had primary hepatic cancer had a 3.5 times higher odds for PVT (95 % CI 2.1-5.8) than cancer-free cirrhosis patients, with a PVT prevalence of more than 14 %. Secondary hepatic malignancy was associated with 4.9 times increased odds (95 % CI 3.8 - 6.2). Within this group, pancreatic cancer patients had a 6.7 times higher odds for PVT (95 % CI: 3.4 - 13.1) than patients with liver metastatic disease from colorectal cancer.
| Patient category | n (%) | PVT (%) | O.R. (95% C.I.)1 | O.R. (95% C.I.) | ||
| P value | P value | |||||
| Cirrhosis | 1193 (5.0) | 72 (6.0) | 7.9 (6.0 - 10.5) | < 0.001 | ||
| with primary hepatic cancer | 182 | 26 (14.3) | 17.1 (11.1 - 26.4) | < 0.001 | 3.5 (2.1 - 5.8) | < 0.001 |
| without primary hepatic cancer | 1011 | 46 (4.5) | 5.2 (3.7 - 7.2) | < 0.001 | 12 | |
| Primary hepatobiliary cancer | 698 (2.9) | 59 (8.5) | 10.8 (8.0 - 14.7) | < 0.001 | ||
| hepatic carcinoma | 392 | 38 (9.7) | 11.5 (8.0 - 16.5) | < 0.001 | ||
| with cirrhosis | 182 | 26 (14.3) | 17.1 (11.1 - 26.4) | < 0.001 | 2.8 (1.3 - 5.6) | 0.004 |
| without cirrhosis | 210 | 12 (5.7) | 5.8 (3.2 - 10.6) | < 0.001 | 12 | |
| extrahepatic biliary / gall bladder carcinoma | 313 | 21 (6.7) | 7.2 (4.5 - 11.4) | < 0.001 | ||
| Secondary hepatic malignancy | ||||||
| from all tumours | 3446 (14.5) | 113 (3.3) | 4.9 (3.8 - 6.2) | < 0.001 | ||
| from pancreatic carcinoma | 312 | 36 (11.5) | 13.9 (9.6 - 20.2) | < 0.001 | 5.2 (3.4 - 7.8) 3 | < 0.001 3 |
| gastric carcinoma | 316 | 18 (5.7) | 5.9 (3.6 - 9.7) | < 0.001 | 1.9 (1.2 - 3.2) 3 | 0.019 3 |
| colorectal carcinoma | 637 | 13 (2.0) | 2.0 (1.1 - 3.5) | 0.028 | 0.5 (0.3 - 1.0) 3 | 0.063 3 |
| Myeloproliferative disorders | 231 (1.0) | 7 (3.0) | 3.0 (1.4 - 6.3) | 0.012 | ||
| All patients | 23796 (100) | 254 (1.0) | ||||
Among the patients with PVT, the highest rates of complications related to portal hypertension were found in the subgroup with concomitant cirrhosis: 62 % had developed ascites, 58 % had oesophageal varices and 47% terminal gastrointestinal bleeding (Table 3). Corresponding figures in all PVT patients were 43 %, 19 % and 25 % (P < 0.001 for all three comparisons), whereas of the 36 patients where no cause of PVT could be identified at autopsy only a minority had these signs of portal hypertension: ascites was only present in 6 (17 %; P < 0.001), oesophageal varices in 2 (6 %; P = 0.036) and only 3 (8 %; P = 0.020) had developed gastrointestinal bleeding.
| Patient category | n | n | Complication | |||||
| Ascites | Oesophageal varices | Gastrointestinal bleeding | ||||||
| n (%) | P value 2 | n (%) | P value 2 | n (%) | P value 2 | |||
| Cirrhosis 1 | 72 | 45 (62) | <0.001 | 42 (58) | <0.001 | 34 (47) | <0.001 | |
| with primary hepatic cancer | 26 | 21 (81) | 13 (50) | 7 (27) | ||||
| without primary hepatic cancer | 46 | 24 (52) | 29 (63) | 27 (59) | ||||
| Primary hepatobiliary cancer 1 | 59 | 34 (58) | 0.016 | 15 (25) | 0.18 | 15 (25) | 0.72 | |
| without cirrhosis | 33 | 13 (39) | 2 (6) | 8 (24) | ||||
| Secondary hepatobiliary malignancy 1 | 111 | 46 (41) | 0.61 | 4 (4) | <0.001 | 15 (14) | 0.002 | |
| Myeloproliferative disorders 1 | 7 | 4 (57) | 0.47 | 2 (29) | 0.62 | 2 (29) | 0.67 | |
| Major abdominal infection / inflammation 1 | 25 | 12 (48) | 0.67 | 1 (4) | 0.06 | 5 (20) | 0.81 | |
| No cause identified | 36 | 6 (17) | <0.001 | 2 (6) | 0.036 | 3 (8) | 0.02 | |
| All patients with PVT | 254 | 110 (43) | 48 (19) | 59 (25) | ||||
Our finding of an overall risk in the general population of 1 % to develop PVT during lifetime does not corroborate the concept of PVT being a rare disease. The figure might even be an underestimation, since it cannot be ruled out that in some cases of partial thrombosis earlier in life a spontaneous resolution may have occurred. It should therefore be relevant to view these patients with renewed interest.
Among the major causes of PVT in adults, cirrhosis is generally named the most common, followed by neoplasia[5-6]. In this study the order of magnitude was reversed, with cancer in two thirds of the patients. By nature of being an autopsy study it is a selection of the sickest patients, and in some of these the thrombosis may be a late event in advanced cancer disease. Discrepancies in proportion of cirrhosis patients compared with previously published case series might also reflect patient selection and ascertainment bias resulting from an increased diagnostic activity in high-risk categories, but one should also consider the impact of disease duration. An intriguing finding was the heterogeneity of risk within the group of metastatic malignancy, with a significantly higher risk with pancreatic cancer in comparison with other major gastrointestinal adenocarcinomas.
When assessing the risk for a patient with a certain condition to develop an infrequent complication like PVT, knowledge from case series on the distribution of causes to that complication may be helpful and is sometimes the only available information. It may be misguiding, though. In this study, secondary malignancies accounted for a twice as large fraction of PVT cases as primary hepatobiliary cancer, but the actual risk of PVT in patients with secondary malignancies was less than half of that associated with primary hepatic cancer. In patients with both cirrhosis and hepatic cancer the risks appeared to be additive resulting in a prevalence of 14%. Whether increased diagnostic and therapeutic activities would be effective to reduce this rate is to be considered for further studies. A recent study, though, indicates that even hepatocellular cancer patients, who have developed portal vein tumour thrombosis, may benefit from an active treatment if timely diagnosed with respect to hepatic function[16].
In one sixth of the PVT patients no explanation could be found despite the thorough examination. This group differed from the other patients by a low frequency of complications typical to portal hypertension. A cross-sectional study like the present has obvious limitations for assessment of previous diseases, and this group may harbour some cases of juvenile PVT, though recent longitudinal studies indicate that PVT is an infrequent complication to umbilical vein sepsis, and in the western world the risk of this complication to umbilical vein catheterisation has declined considerably over time[5,17-18]. Similarly, it cannot be ruled out that the thrombosis in the odd case might have been caused by surgical trauma not evident from the recent patient history. With increasing possibilities to detect various trombophilic disorders the proportion of non-explainable, so called idiopathic PVT has gradually become smaller[5,10,19-22]. The present study does not allow an investigation of markers of the haemostatic system, but it is not unreasonable that the PVT in some of these cases may be an expression of a hypercoagulability state.
Autopsy rates have declined considerably also in Malmö, following changes in legislation, and it is unlikely that a similar study will ever be performed again. With regards to the setting in time, some limitations should be discussed. Apart from aspects of the design previously viewed, the potential effects on the epidemiology of an increasing use of antithrombotic therapies should be considered. The study does not allow an assessment of actual medications, but even today the majority of these conditions would not warrant primary or secondary anticoagulant prophylaxis according to guidelines[23].
From this first population-based study we conclude that PVT is more common than previously indicated by clinical series. The greatly increased risk in patients with cirrhosis and hepatic carcinoma should warrant an increased awareness in these patients. While anticoagulation therapy might be considered for certain patient categories, this remains to be evaluated in prospective studies.
| 1. | Ohnishi K, Okuda K, Ohtsuki T, Nakayama T, Hiyama Y, Iwama S, Goto N, Nakajima Y, Musha N, Nakashima T. Formation of hilar collaterals or cavernous transformation after portal vein obstruction by hepatocellular carcinoma. Observations in ten patients. Gastroenterology. 1984;87:1150-1153. [PubMed] |
| 2. | Brown KM, Kaplan MM, Donowitz M. Extrahepatic portal venous thrombosis: frequent recognition of associated diseases. J Clin Gastroenterol. 1985;7:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, Musha H, Takashi M, Suzuki N, Shinagawa T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. 1985;89:279-286. [PubMed] |
| 4. | Belli L, Romani F, Riolo F, Rondinara G, Aseni P, Di Stefano M, Contorni L, Bini M. Thrombosis of portal vein in absence of hepatic disease. Surg Gynecol Obstet. 1989;169:46-49. [PubMed] |
| 5. | Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med. 1992;92:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 174] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE. Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut. 2001;49:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Tanaka H, Horie Y, Idobe Y, Murawaki Y, Suou T, Kawasaki H. Refractory ascites due to portal vein thrombosis in liver cirrhosis--report of two cases. Hepatogastroenterology. 1998;45:1777-1780. [PubMed] |
| 8. | Webb LJ, Sherlock S. The aetiology, presentation and natural history of extra-hepatic portal venous obstruction. Q J Med. 1979;48:627-639. [PubMed] |
| 9. | Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, Binda T, Varet B, Rueff B, Benhamou JP. Etiology of portal vein thrombosis in adults. A prospective evaluation of primary myeloproliferative disorders. Gastroenterology. 1988;94:1063-1069. [PubMed] |
| 10. | Janssen HL. Changing perspectives in portal vein thrombosis. Scand J Gastroenterol Suppl. 2000;69-73. [PubMed] |
| 11. | Witte CL, Brewer ML, Witte MH, Pond GB. Protean manifestations of pylethrombosis. A review of thirty-four patients. Ann Surg. 1985;202:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sternby NH. Atherosclerosis in a defined population. An autopsy survey in Malmö, Sweden. Acta Pathol Microbiol Scand. 1968;Suppl 194:5+. [PubMed] |
| 13. | Bergqvist D, Lindblad B. A 30-year survey of pulmonary embolism verified at autopsy: an analysis of 1274 surgical patients. Br J Surg. 1985;72:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Incidence of acute thrombo-embolic occlusion of the superior mesenteric artery--a population-based study. Eur J Vasc Endovasc Surg. 2004;27:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Ogren M, Bergqvist D, Eriksson H, Lindblad B, Sternby NH. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23 796 consecutive autopsies. Eur Heart J. 2005;26:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Fan J, Zhou J, Wu ZQ, Qiu SJ, Wang XY, Shi YH, Tang ZY. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215-1219. [PubMed] |
| 17. | Yadav S, Dutta AK, Sarin SK. Do umbilical vein catheterization and sepsis lead to portal vein thrombosis A prospective, clinical, and sonographic evaluation. J Pediatr Gastroenterol Nutr. 1993;17:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Guimarães H, Castelo L, Guimarães J, Cardoso A, d'Orey C, Mateus M, Almeida A, Amil Dias J, Ramos I, Teixeira Santos N. Does umbilical vein catheterization to exchange transfusion lead to portal vein thrombosis. Eur J Pediatr. 1998;157:461-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Gürakan F, Eren M, Koçak N, Yüce A, Ozen H, Temizel IN, Demir H. Extrahepatic portal vein thrombosis in children: etiology and long-term follow-up. J Clin Gastroenterol. 2004;38:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hirohata Y, Murata A, Abe S, Otsuki M. Portal vein thrombosis associated with antiphospholipid syndrome. J Gastroenterol. 2001;36:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 455] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Violi F, Ferro D, Basili S, Lionetti R, Rossi E, Merli M, Riggio O, Bezzi M, Capocaccia L. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost. 1997;77:44-47. [PubMed] |
| 23. | Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S-400S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2163] [Cited by in RCA: 1979] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Zhang JZ E- Editor Ma WH