Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2040
Revised: October 25, 2005
Accepted: November 10, 2005
Published online: April 7, 2006
AIM: To construct two kinds of anti-gastrin immunogen based on P64K protein from Neisseria meningitids and to compare their immunogenic effect.
METHODS: G17P64K gene was cloned and ligated into pET28a plasmid, then transformed into BL21(DE3). After inoculation of LB medium and IPTG induction, the recombinant protein was solubly expressed at a high level.The purification of G17P64K fusion protein was similar to that of P64K. An initial step of purification consisting of 30 % saturated ammonium sulfate precipitation was done. Additional fine optimizations included phenyl-sepharose, G200 Sephadex gel filtration and Q-sepharose anion exchanger chromatography. Highly purified protein was obtained and sequenced at the N-terminal amino acid residues. Polypeptide was synthesized by Fmoc solid phase chemical method and cross-linked to carrier protein P64K and DT mutant by MBS method and then the rabbit anti-gastrin 17 antibody was prepared by immunizing rabbit with cross-linked and fused protein. The titer and the activity in vitro of antibody were assessed.
RESULTS: G17P64K gene and the recombinant bacteria were obtained. After four steps purification, protein sample that has the purity above 90 % was achieved. At the 84th day after the first immunization, the titer of antibody against cross-linked protein reached 51 200. Evaluation of the antibody in vitro manifested that it had a high inhibitory activity on the growth of tumor cell SW480.
CONCLUSION: The P64K-polypeptide cross-linked immunogen immunized rabbit and achieved a higher titer antibody against gastrin 17 than the G17P64K fusion protein immunogen, which could inhibit the growth of the tumor cell SW480.
- Citation: Xiong XH, Zhao HL, Xue C, Zhang W, Yang BF, Yao XQ, Liu ZM. Construction and evaluation of anti-gastrin immunogen based on P64K protein. World J Gastroenterol 2006; 12(13): 2040-2046
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2040.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2040
The peptide hormone gastrin, released from the G cells of the antrum and duodenum, is known to stimulate the secretion of gastric acid and act as a trophic factor within gastrointestinal tract[1-3]. In recent years evidence has accumulated that gastrin is a growth factor for gastrointestinal tumors, such as gastric cancer, colon cancer, and pancreatic cancer[3-6]. Therefore it has emerged as a potential anticancer target[7].
There are several forms of gastrin; the precursor molecule preprogastrin is cleaved by an endopeptidase to progastrin, which is further processed to glycine-extended gastrin 34 and to gastrin 34[8]. These 35- and 34-amino acid peptides can be cleaved to form glycine-extended gastrin 17 and gastrin 17 respectively. Cells with gastrin receptors can respond to mature amidated forms of gastrin, as well as precursor forms, particularly glycine-extended gastrin 17. It has become clear that in cancer cells originating in various parts of the gut, the gastrin gene and the genes that encode the gastrin receptors are switched on at a very early stage of oncogenesis. Scientists have demonstrated that the glycine-extended gastrin 17 and gastrin 17 can promote cancer cell division via an autocrine or endocrine pathway[9]. A unique B cell epitope to glycine-extended gastrin 17 and gastrin 17, which is not found on gastrin 34 and CCK, consisting of the 9-amino acid stretch at the amino-terminal end of the molecule, has been identified and mapped. The immunogens comprising this unique epitope result in high levels of anti-gastrin 17 antibodies that do not cross-react with gastrin 34 and CCK, which share with gastrin 17 a common receptor to carry out their physiologic function.
P64K protein isolated from pathogenic bacterium Neisseria is found in the outer membrane of the cell and well recognised in sera from individuals convalescent from meningococcal disease or vaccinated with VA-MENGOC-BC®, a Cuban antimeningococcal vaccine based on outer membrane vesicles[10]. Its high molecular mass and strong immunogenicity have made it a suitable carrier protein for weak immunogens. Recent studies have manifested that P64K protein has a better immune enhancing effect than conventional carrier protein such as BSA, TT[11]. The B cell epitope of gastrin 17 was cross-linked or fused via a peptide spacer to P64K to comprise an immunogen against gastrin 17.
P64K gene and protein were conserved in our laboratory. DH5α, BL21(DE3) were purchased from BioDev (Beijing China). Restriction endonucleases, polymerase, T4 DNA ligase and DL2000 were from Takara (Dalian,China). GE-NECLEAN II kit was ordered from Q.BIOgene (Morgan Irvine,USA). SV Minipreps DNA purification system was provided by Promega (Madison,USA). DT mutant (CRM197) was purchased from Sigma(Ronkonkoma, USA). Polypeptide was synthesized and cross-linked in Institute of Basic Medical Sciences (Academy of Military Medical Sciences, Beijing, China). Low molecular weight calibration kit for SDS electrophoresis was purchased from Amersham Biosciences(Beijing,China). Goat anti-rabbit IgG-HRP was from Bio-LAB(Beijing China). New Zealand white rabbits and BALB/C nude mice were supplied by Laboratory of Animal Center (Academy of Military Medical Sciences, Beijing, China).
SW480 is a human colonic epithelial tumor cell. It could be stimulated to proliferate by gastrin via an autocrine or endocrine pathway and inhibited by gastrin inhibitor[12,13]. It was obtained from The Cell Center of Basic Medical Sciences(Chinese Academy of Medical Sciences, Beijing, China). Cell lines were grown in DMEM (GIBCO-BRL) supplemented with 10 % heat-activated fetal bovine serum(FBS) in a humidified incubator at 37 °C in an atmosphere of 5 % CO2.
Construction of recombinant expression plasmid: Cloning of gene, isolation of plasmid and all other molecular biology procedures were carried out according to the standard procedures published. P64K gene was cloned from Neisseria meningitids and gastrin17 B cell epitope was designed into 5’ upperstream primer[14]. G17P64K gene was cloned by PCR amplification under the following conditions:30 cycles of 94°C for 1 min, 50 °C for 1 min and 72 °C for 2 min with one additional cycle for 10 min at 72 °C. The reaction components were: 1μg of P64K DNA; 50 pmol of primer 1 (5’-CATGCCATGGAAGGCCCTTGGCTTGAAGAGGAAGAATCTTCACCCCCTCCGCCGCTTTAGTTGAATTGAAAGTG-3’) and primer 2 (5’-GGGAATTCTTATTTTTTCTTTTGCGGAG-3’); 200 μmol/L of each deoxynucleotide triphosphates (dNTPs); PCR buffer (10 mmol/L KCl, 20 mmol/L Tris-HCl pH 8.8, 10 mmol/L (NH4)2SO4, 2 mmol/L MgSO4, 0.1 %Triton X-100; double distilled water to a final volume of 50 μL and 1 unit per reaction of Pyrobest DNA polymerase. PCR product was purified by agarose gel electrophoresis, digested by NcoI and EcoRI, then ligated into the pET28a plasmid digested with the same enzymes. The ligation products were then transformed into E.coli DH5α. Positive clones were selected by PCR using the conditions described above and subjected to double-stranded DNA sequencing with T7 sequencing primer according to the manufacturer’s specifications. Sequence processing was done with the DNAStar software.
Expression of recombinant protein in shake flask cultures: The strain E.coli BL21 (DE3) was transformed with G17P64K recombinant plasmid, which was also transformed with plasmid (negative control). A transformed colony from each construct was inoculated into 20 mL Luria Bertani (LB) cultures. Bacteria were grown in a shaker at 37°C. After measurement of the optical density at 600 nm (OD600), IPTG was added to induce recombinant protein synthesis considering OD600 of 0.5. Cell culture was allowed to continue incubation for 4 h. Samples were collected and centrifuged at 10 000 g for 10 min at 4 °C. The bacterial pellet was washed twice with 10 mmol/L Tris and 1mmol/L EDTA, pH8 (TE), and then resuspended in 1 mL TE. Cells were destructed by sonication for 15 min. After centrifugation of the lysate at 27 000 g for 30 min at 4°C, supernatants and pellets from destruction were collected. All protein samples were subjected to 15 % SDS-PAGE according to the standard procedure of Laemmli. Protein bands were visualized by staining with Coomassie brilliant G-250. Gels were scanned and the purity was estimated by densitometry using the Molecular Analysis software.
Production of recombinant protein in bioreactors: The colony containing recombinant plasmid was inoculated into 5 mL cultures of LB containing kanamycin(30 μg/mL). Cells were grown in a shaker at 37 °C to an OD600 of 0.8. The sample was transferred into 200 mL LB culture and continued to grow for 12 h at 37 °C with shaking (200 rpm). This culture was used to inoculate 3 L of rich media supplemented with 30 μg/mL kanamycin. When OD600 reached 20, expression of G17P64K gene was induced by addition of IPTG to a final concentration of 1 mmol/L and allowed to take place at 37 °C for 7 h. One milliliter of the bacteria was taken out at 0, 1, 2, 3, 4, 5, 6 and 7 h after induction. Cells were harvested by centrifugation at 10 000 g for 20 min and stored at -20 °C. Five microliters of bacteria were electrophoresed on a 15 % SDS-PAGE for evaluation of protein expression.
Purification of the recombinant protein: The supenatant obtained after centrifugation of the lysate was precipitated by adding solid ammonium sulfate to 20 %, 30 %, 40 %, 50 % saturation respectively. And the condition of the most optimal purification effect was ascertained. The pellet was resuspended after centrifugation at 27 000 g for 20 min at 4 °C. The resuspended solution was applied to the following chromatography steps similar to P64K.
The first stage was a phenyl-sepharose hydrophobic interaction column. After the sample was loaded, the column was washed with loading buffer(2.0 M ammonium sulfate, 20 mmol/L Tris-HCl, 1 mmol/L EDTA, pH7.2) and eluted with a 1.5-0 mol/L ammonium sulfate gradient. G17P64K protein was eluted as a major peak in about 0.8M ammonium sulfate and G17P64K-containing fractions were pooled by adding solid ammonium sulfate to 100 % saturation. The second step was a G200 Sephadex column. The concentrated sample was loaded on the column, reequilibrated and eluted with PBS. G17P64K was eluted in the major peak and both sides contained only small amounts of contaminants. The last chromatography step was a Q-Sepharose anion exchange column. The sample from the previous G-200 sephadex column was diluted 10 times and loaded onto the anion exchange column. After washing the column with loading buffer (20 mmol/L KPB, 5mmol/L NaCl, 1mmol/L EDTA, pH6.0), G17P64K was eluted with 5-500 mmol/L NaCl gradient. In this step, the G17P64K-containing peak was the only major peak. The samples were subjected to 15% SDS-PAGE under denaturing conditions as previously described.
Chemical synthesis and crosslink of polypeptide: Po-lypeptide with the following amino acid sequences were synthesized: pyro-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Gly-Gly-Gly-Gly-Ser-Cys. Synthetic polypeptide contained a B-cell epitope consisting of residues 1 to 9 of gastrin 17, a five amino acid peptide spacer, and an N-terminal cysteine. It was produced by Fmoc solid-phase synthetic chemical method and its purity was detected by high-performance liquid chromatography(HPLC) analysis method. The polypeptide was conjugated to amino group present on a carrier protein such as P64K and CRM-197(DT mutant) via the terminal peptide cysteine residue utilizing heterobifunctional linking agent-m-maleimidobenzoyl-N-hydroxysuccinimide ester(MBS). After desalting and concentrating over a PM 10 ultrafiltration membrane, the conjugate was lyophilized and stored desiccatedly at -20°C until use.
Preparation of rabbit anti-gastrin 17 antibody: New Zealand rabbits were immunized with the immunogens including synthetic polypeptide, G17P64K recombinant protein, polypeptide-DT conjugate and polypeptide-P64K conjugate. Four groups of 2 rabbits were immunized with one of the immunogens. Each animal was injected subcutaneously with 1.5 mL of immunogen. An additional group of 2 rabbits was immunized with P64K protein as a blank immunization control. Each group of rabbit was immunized as shown in Table 1. Rabbits were vaccinated by subcutaneous injection of immunogens emulsified with CFA/PBS for the first immunization and IFA/PBS for the subsequent 2 booster injections at 3-wk intervals. Blood samples were collected from rabbits at wk 0, 2, 5, 8 and 12.
| Polypeptide(mg/rabbit) | G17P64Kprotein(mg/rabbit) | G17(9) :P64K(mg/rabbit) | G17(9) :DT(mg/rabbit) | P64K protein(mg/rabbit) |
| 0.25 | 2.0 | 0.5 | 0.5 | 0.25 |
Detection of antibody titer: Antibodies levels in sera were determined by enzyme linked immunosorbent assay (ELISA). To detect anti-gastrin 17 antibodies titer, 96-well plates were coated with 200 μL/well of corresponding pep-tide(10 μg/mL) in carbonate buffer(0.05 mol/L NaCO3, pH 9.6) for 2 h at 37 °C. Bovine serum albumin (2 %) was used as a blocking reagent. After three washes in /0.05 % PBS Tween 20 (PBST), 100 μL of serial dilution of each serum (starting dilution 1:100) was added to the plates, which were incubated for 2 h at 37 °C. All sera were analyzed in four replicates. Serum anti-gastrin 17 antibody levels were expressed as their absorbance (492 nm) values in ELISA and used for statistical analysis.
Evaluation of the antibody in vitro: SW480 cells were seeded into 96 flat bottomed well plates(Costar) at a concentration of 5×104 per well in a 100 μL volume in the serum free medium. Affinity-purified immunoglobulins(IgG) raised against gastrin 17 and normal rabbit control IgG were normalized to standard protein concentration of 0.5 mg/mL. IgG dilutions from 1/2 to 1/256 were prepared in serum free medium and added to the cells in 100 μL volume per well. Each condition was performed with four replicates. Cell proliferation was assessed after 72 h by the methylthiazoyl tetrazolium(MTT) assay.
Statistics evaluation of in vitro data was performed by a one way analysis of variance on the SPSS package.
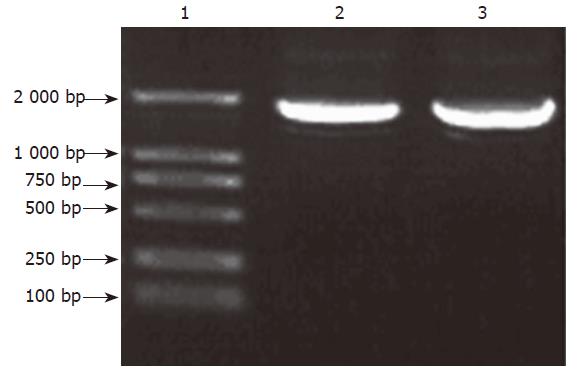
After amplification of G17P64K gene by PCR, there was a band of about 1.8kb by agarose gel electrophoresis(Figure 1). The fragment was purified, digested by NcoI and EcoRI and cloned into the pET28a. The resulting plasmid was designated as pET28a-G17P64K. Sequencing revealed that the G17P64K gene was fused to the correct reading frame with the coding sequence.
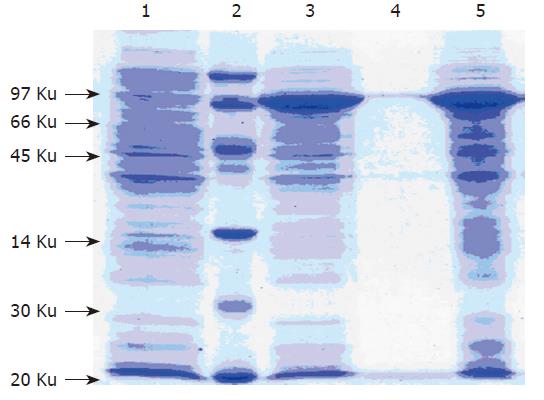
SDS-PAGE was carried out with the sample from BL21(DE3), which transformed with pET28a- G17P64K (Figure 2). An approximate 70KD protein was solubly expressed in BL21(DE3) and accounted for about 30 % of the total cellular protein.
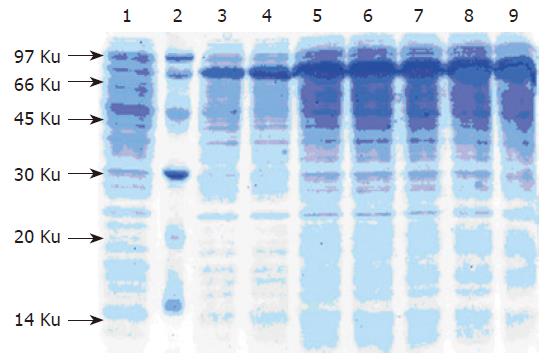
Recombinant bacteria were grown in 5-L fermentors to investigate whether the G17P64K protein could be expre-ssed and the yields could be amplified using a scale-up cultivation. The 5-L fermentors strategy allowed cultures to grow to final OD600 of 33. At 5-L fermentors culture of 5 h, induction of the T7 promotor and production of G17P64K protein was initiated. The G17P64K protein was gradually increased per OD600 until the end of fermentation (Figure 3).
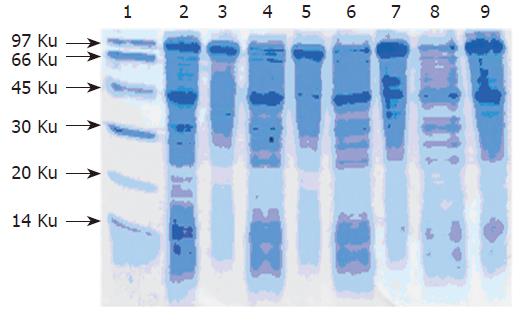
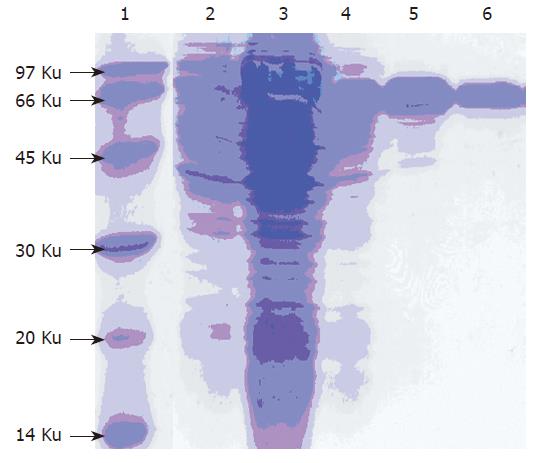
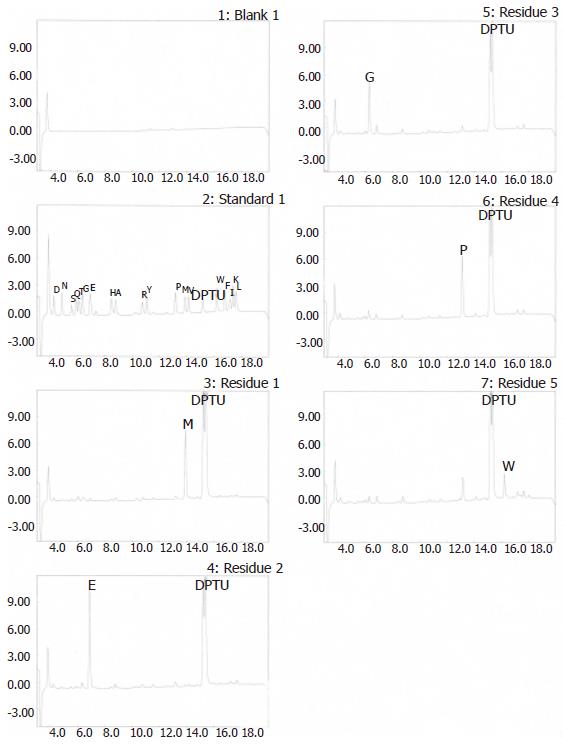
The G17P64K production system described in this work may permit the protein to be purified using commercially available, conventional method. SDS-PAGE manifested that 30 % saturation of ammonium sulfate could most efficiently remove the contaminants (Figure 4). The first step consisted of a phenyl-sepharose hydrophobic interaction column chromatography and used a high con-centration to enhance G17P64K capture. A G200 Sep-hadex column chromatography was used next to further minimize host cell protein contaminants, followed by a Q-Sepharose anion exchanger column chromatography to help eliminate the residual contaminants. The results of this purification scheme are shown in Figure 5. After four steps of purification, the final product was judged to be more than 90 % of purity. And the result of N-terminal amino acid sequencing (MEGPW) was in accordance to the anticipated sequence (Figure 6).
Polypeptide was synthesized by Fmoc solid-phase synthetic chemical methods. The total weight of synthetic polypeptide was determined to be 60mg and the purity was detected to be 66.84 % by HPLC (Figure 7). The synthetic peptide was conjugated to carrier protein via MBS cross-linking reagent. The conjugate was lyophilized and stored desiccatedly at -20 °C until use.
Anti-gastrin 17 antibody levels in rabbit sera were deter-mined by ELISA (Table 2). High levels of antibody were obtained against synthetic polypeptide conjugated to protein carriers DT and P64K. The result also revealed that the immunogenicity of G17P64K fusion protein was as weak as the single synthetic peptide.
| Polypeptid | G17P64Kprotein | G17(9) :DT | G17(9) :P64K | |
| D 0 | 0 | 0 | 0 | 0 |
| D14 | 100 | 100 | 800 | 800 |
| D35 | 800 | 800 | 6400 | 6400 |
| D54 | 3200 | 3200 | 25600 | 25600 |
| D84 | 6400 | 6400 | 51200 | 51200 |
MTT uptake was used to measure in vitro proliferation which assesses the oxidative ability of the cell to convert a soluble tetrazolium compound into an insoluble colored crystalline product which can be dissolved and assessed colorimetrically. This has previously been shown to correlate with direct cell counts in many cases. The characterization of SW480 has revealed that endocrine/autocrine gastrin–mediate pathways may be operational in the growth of the cells. Figure 8 shows the MTT uptake of SW480 in the presence of increasing dilutions of rabbit ani-gastrin 17 IgG. In experiment, the growth of SW480 was significantly reduced by treatment with rabbit anti-gastrin 17(9) : DT and anti-gastrin 17(9) : P64K from 1/1 to 1/16 dilution, and modestly inhibited at dilutions of 1/32 and 1/64 (P < 0.05, one way analysis of variance). The effect of rabbit anti-G17(9) : P64K treatment on the in vitro growth of SW480 was not significantly different from rabbit anti-G17(9) : DT from 1/1 to 1/64.
Immunization against specific cancer promoting hormones may be useful in the treatment and prevention of cancer. Gastrin has been identified as the central trophic factor for gastrointestinal cancers and has therefore emerged as a potential anticancer target. There are many optional inhibitors of gastrin production[15]. Gastrin-receptors an-tagonists have been evaluated, but failed to produce any survival benefit in clinical trials[16-18]. Anti-gastrin antibody was demonstrated to be able to bind to gastrin peptides and prevent their interaction with gastrin receptors in preclinical research. And in vivo animal studies showed that infusion of anti-gastrin antibodies could inhibit the growth of human colorectal xenografts[19-22]. However, passive infusions of antibodies have serious drawbacks for the patient. Antibodies given passively to patients are not long-lasting and 1 or 2-h infusion would be required every week. In addition, antibody titer would have to be in excess of those required to neutralize normal gastrin secretion so that tumor-associated gastrin would be tar-geted. Active immunization was likely to work better. A novel immunotherapy has now completed phase III clinical trials of “Gastrimmune” as monotherapy in patients with pancreatic cancer and reported positive res-ults[23-25]. Aphton Corp. has filed for marketing approval in Australia, Canada and New Zealand in 2004. Therefore targeting gastrin as an active-immunotherapeutic approach is appropriate.
There are several forms of gastrin. The glycine-extended gastrin 17 and gastrin 17 are the central trophic factor of cancer cell, while gastrin 34 and CCK are necessary for human body to maintain normal operation. A unique B cell epitope to glycine-extended gastrin 17 and gastrin 17 that is not found on gastrin 34 and CCK, consisting of the 9-amino acid stretch at the amino-terminal end of the molecule, has been identified and mapped. The immunogens comprising this unique epitope result in high levels of anti-gastrin 17 antibodies that do not crossreact with gastrin 34 and CCK, and could effectively decrease the side-effect of vaccine.
An important drawback for the use of the conventional carrier protein in different conjugate vaccines is carri-erinduced hapten-specific suppression. It has been demon-strated when mice, presensitized with keyhole limpet hemocyanin (KLH) and subsequently vaccinated with KLH-dinitrophenol(DNP) conjugates, elicited a reduced anti-DNP IgG response[26]. Being a novel carrier protein, P64K protein has its superiority to BSA, TT and DT. This study evaluated the effect of P64K presensitization on its ability as a carrier, by comparing the hapten-specific humoral immune response elicited in rabbit. It proved that P64K protein can help to activate humoral immune system and enhance immune reaction against gastrin 17 B cell epitope as DT.
There are two problems for the immunogen comprising G17 recombinant protein associated with the low level of anti-gastrin 17 antibody because the immunogen is made up of single synthetic polypeptide. First, the excess meth-ionine at the N-terminal of the G17P64K recombinant protein remodels the conformation of the B cell epitope of gastrin 17, and thereof disturbs the recognition of the B cell. Second, polypeptide could provide more B-cell epitope than the G17P64K recombinant protein in the experiment of preparation of anti-gastrin 17 antibody.
A series of tests should be done to assess the inhibitory effect and specificity of the P64K-gastrin immunogen, including the in vivo experiment and the adjustment between the inhibitory effect and the gastrin 17 levels. Furthermore, if there were a suitable cell line, an in vivo experiment could be done directly, but not human-rabbit-mice conversion. Another test is the quality control of the crosslink immunogen of P64K and synthetic peptide. Other kinds of cancer cell line should be considered.
We acknowledge Professor Ling-Shuang Xiong for her in-structions. We would like to thank He-Ping Pan for the technical assistance. We also thank Dr. Xiang He for her support in this project.
| 1. | Modlin IM, Kidd M, Marks IN, Tang LH. The pivotal role of John S. Edkins in the discovery of gastrin. World J Surg. 1997;21:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Urushidani T, Forte JG. Signal transduction and activation of acid secretion in the parietal cell. J Membr Biol. 1997;159:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Wang Z, Chen WW, Li RL, Wen B, Sun JB. Effect of gastrin on differentiation of rat intestinal epithelial cells in vitro. World J Gastroenterol. 2003;9:1786-1790. [PubMed] |
| 4. | Smith JP, Verderame MF, Ballard EN, Zagon IS. Functional significance of gastrin gene expression in human cancer cells. Regul Pept. 2004;117:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhou JJ, Chen ML, Zhang QZ, Hu JK, Wang WL. Coexpression of cholecystokinin-B/gastrin receptor and gastrin gene in human gastric tissues and gastric cancer cell line. World J Gastroenterol. 2004;10:791-794. [PubMed] |
| 6. | Jang JY, Kim SW, Ku JL, Park YH, Park JG. Presence of CCK-A, B receptors and effect of gastrin and cholecystokinin on growth of pancreatobiliary cancer cell lines. World J Gastroenterol. 2005;11:803-809. [PubMed] |
| 7. | Baldwin GS, Shulkes A. Gastrin as an autocrine growth factor in colorectal carcinoma: implications for therapy. World J Gastroenterol. 1998;4:461-463. [PubMed] |
| 8. | Wiborg O, Berglund L, Boel E, Norris F, Norris K, Rehfeld JF, Marcker KA, Vuust J. Structure of a human gastrin gene. Proc Natl Acad Sci U S A. 1984;81:1067-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Koh TJ, Chen D. Gastrin as a growth factor in the gastrointestinal tract. Regul Pept. 2000;93:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Silva R, Menéndez T, Alonso LM, Iglesias E, Musacchio A, Leal MJ, Alvarez A, Coizeau E, Martín A, Herrera L. Characterisation of the lpdA gene from Neisseria meningitidis by polymerase chain reaction, restriction fragment length polymorphism and sequencing. FEMS Microbiol Lett. 1999;174:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Costantino P, Viti S, Podda A, Velmonte MA, Nencioni L, Rappuoli R. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Bin X, He SW, Wang DK. Effects and clinical sig-nificance of vincristing in gastrin-stimulating cell proliferation on human colonic cancer cell line SW480. Zhongguo Puwai Jichu Yu Linchuang. 2000;7:3-5. |
| 13. | He SW, Zhao YM, Shen KQ. Promoting effects of gastrin on transplanted human colonic carcinoma in nude mice. Disan Junyi Daxue Xuebao. 2000;2:112-114. |
| 14. | Xiong XH, Zhao HL, Xue C, Zhang W, Yang BF, Liu ZM. The soluble expression in E.coli and purification of a new carrier protein, P64K. Biotechnol Lett. 2004;15:231-234. |
| 15. | Smith AM, Watson SA. Review article: gastrin and colorectal cancer. Aliment Pharmacol Ther. 2000;14:1231-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Attoub S, Moizo L, Laigneau JP, Alchepo B, Lewin MJ, Bado A. YM022, a highly potent and selective CCKB antagonist inhibiting gastric acid secretion in the rat, the cat and isolated rabbit glands. Fundam Clin Pharmacol. 1998;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Takinami Y, Yuki H, Nishida A, Akuzawa S, Uchida A, Takemoto Y, Ohta M, Satoh M, Semple G, Miyata K. YF476 is a new potent and selective gastrin/cholecystokinin-B receptor antagonist in vitro and in vivo. Aliment Pharmacol Ther. 1997;11:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | He SW, Shen KQ, He YJ, Xie B, Zhao YM. Regulatory effect and mechanism of gastrin and its antagonists on colorectal carcinoma. World J Gastroenterol. 1999;5:408-416. [PubMed] |
| 19. | Hoosein NM, Kiener PA, Curry RC, Rovati LC, McGilbra DK, Brattain MG. Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human colon carcinoma cell lines. Cancer Res. 1988;48:7179-7183. [PubMed] |
| 20. | Watson SA, Michaeli D, Grimes S, Morris TM, Crosbee D, Wilkinson M, Robinson G, Robertson JF, Steele RJ, Hardcastle JD. Anti-gastrin antibodies raised by gastrimmune inhibit growth of the human colorectal tumour AP5. Int J Cancer. 1995;61:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Watson SA, Michaeli D, Morris TM, Clarke P, Varro A, Griffin N, Smith A, Justin T, Hardcastle JD. Antibodies raised by gastrimmune inhibit the spontaneous metastasis of a human colorectal tumour, AP5LV. Eur J Cancer. 1999;35:1286-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Watson SA, Michaeli D, Grimes S, Morris TM, Robinson G, Varro A, Justin TA, Hardcastle JD. Gastrimmune raises antibodies that neutralize amidated and glycine-extended gastrin-17 and inhibit the growth of colon cancer. Cancer Res. 1996;56:880-885. [PubMed] |
| 23. | Watson SA, Michael D, Justin TA, Grimes S, Morris TM, Robinson G, Clarke PA, Hardcastle JD. Pre-clinical evaluation of the Gastrimmune immunogen alone and in combination with 5-fluorouracil/leucovorin in a rat colorectal cancer model. Int J Cancer. 1998;75:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Smith AM, Justin T, Michaeli D, Watson SA. Phase I/II study of G17-DT, an anti-gastrin immunogen, in advanced colorectal cancer. Clin Cancer Res. 2000;6:4719-4724. [PubMed] |
| 25. | Gilliam AD, Watson SA, Henwood M, McKenzie AJ, Humphreys JE, Elder J, Iftikhar SY, Welch N, Fielding J, Broome P. A phase II study of G17DT in gastric carcinoma. Eur J Surg Oncol. 2004;30:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Herzenberg LA, Tokuhisa T, Herzenberg LA. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
S- Editor Wang XL L- Editor Zhu LH E- Editor Wu M