Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2024
Revised: November 11, 2005
Accepted: November 18, 2005
Published online: April 7, 2006
AIM: To investigate the protective effect of rosuvastatin on ischemia-reperfusion (I-R)-induced small intestinal injury and inflammation in rats, and to determine the effect of this agent on the expression of endothelial nitric oxide synthase (eNOS) protein.
METHODS: Intestinal damage was induced in male Sprague-Dawley rats by clamping both the superior mesenteric artery and the celiac trunk for 30 min, followed by reperfusion for 60 min. Rosuvastatin dissolved in physiological saline was administered intraperitoneally 60 min before ischemia. The severity of the intestinal mucosal injury and inflammation were evaluated by several biochemical markers, as well as by histological findings. The protein levels of eNOS were determined by Western blot.
RESULTS: The levels of both intraluminal hemoglobin and protein, as indices of mucosal damage, were significantly increased in the I-R group compared with those in the sham-operated group. These increases, however, were significantly inhibited by treatment with rosuvastatin in a dose-dependent manner. The protective effects of rosuvastatin were also confirmed by histological findings. Exposure of the small intestine to I-R resulted in mucosal inflammation characterized by significant increases in thiobarbituric acid-reactive substances, tissue-associated myeloperoxidase activity, and the mucosal contents of rat cytokine-induced neutrophil chemoattractant-1 (CINC-1) and tumor necrosis factor-α (TNF-α). These increases in inflammatory parameters after I-R were significantly inhibited by pretreatment with rosuvastatin at a dose of 10 mg/kg. Furthermore, mRNA expression of CINC-1 and TNF-α was increased after I-R, and this increase was also inhibited by rosuvastatin. The mucosal protein levels of eNOS decreased during I-R, but were preserved in rats treated with rosuvastatin.
CONCLUSION: Rosuvastatin inhibits rat intestinal injury and inflammation induced by I-R, and its protection is associated with the preservation of eNOS protein.
- Citation: Naito Y, Katada K, Takagi T, Tsuboi H, Kuroda M, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T. Rosuvastatin reduces rat intestinal ischemia-reperfusion injury associated with the preservation of endothelial nitric oxide synthase protein. World J Gastroenterol 2006; 12(13): 2024-2030
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2024
Intestinal ischemia-reperfusion (I-R) injury is a grave condition resulting from acute mesenteric ischemia, small bowel transplantation, abdominal aortic aneurysm, hemorrhagic, traumatic or septic shock, or severe burns[1,2]. Recent reports have demonstrated that neutrophil infiltration in the intestinal mucosa via neutrophil-endothelial cell interactions plays a significant role in the pathogenesis of I-R-induced intestinal injury because activated neutrophils generate tissue damaging products such as reactive oxygen species, protease, collagenase, and a ferrous iron-ferritin complex [3-5]. Leukocyte accumulation is a complex phenomenon that also involves endothelium-based adhesion molecules as well as leukocyte chemotaxis factors such as interleukin-8 (IL-8). Intercellular adhesion molecules (ICAMs) are normally expressed at a low basal level, but their expression can be enhanced by various inflammatory cytokines such as IL-1 and tumor necrosis factor-α (TNF-α). A variety of cytokines, including TNF-α, interferon-γ and IL-1β, are released from post-ischemic tissues.
Statins, which are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are widely used in the treatment of hyperlipidemia and coronary artery disease [6]. Recently accumulating evidence suggests that statins have anti-inflammatory and endothelial cell protective actions that are independent of their cholesterol-lowering effect [7]. Statins can increase the expression of endothelial nitric oxide synthase (eNOS) by blocking Rho geranylgeranylation [8], and they can also block the lymphocyte function-associated antigen-1 (LFA-1)-ICAM-1 interaction by binding the L-site [9]. Rosuvastatin, a new HMG-CoA reductase inhibitor, has exhibited a more potent affinity for the active site of HMG-CoA reductase than other statins. In addition, the cytoprotective action of rosuvastatin against ischemic injury has been clearly documented [10-14]. Ikeda et al [10], for example, demonstrated that rosuvastatin reduces neutrophil-induced cardiac contractile dysfunction in the isolated ischemic reperfused rat heart. However, to date, the effects of rosuvastatin on intestinal I-R injury have not yet been investigated. In this study, we show that rosuvastatin dramatically attenuates I-R-induced intestinal injury and inflammation. Moreover, rosuvastatin was found to reverse the decrease in eNOS expression after I-R in intestinal mucosa.
All chemicals were prepared immediately prior to use. Rosuvastatin was a gift from AstraZeneca UK, Ltd. (London, UK). Thiobarbituric acid (TBA) and 3,3’,5,5’-tetramethylbenzidine were obtained from Wako Pure Chemical Indust. (Osaka, Japan), and 1,1,3,3-tetramethoxypropane was obtained from Tokyo Kasei (Tokyo, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for rat TNF-α and cytokine-induced neutrophil chemoattractant-1 (CINC-1) were obtained from BioSource International (Camarillo, CA) and Immuno-Biological Laboratories Co., Ltd. (Gunma, Japan), respectively. All other chemicals used were of reagent grade.
Male Sprague-Dawley rats weighing 180-200 g were obtained from Keari Co., Ltd. (Osaka, Japan). The animals were housed at 22 °C in a controlled environment with 12 h of artificial light per day. They were allowed access to rat chow and water ad libitum. The rats were anesthetized with urethane (1 mg/kg, ip). After a midline laparotomy, the celiac and superior mesenteric arteries were isolated near their aortic origins. Intestinal ischemia was induced by clamping both the superior mesenteric artery and the celiac trunk for 30 min, resulting in a total occlusion of these arteries, following our previously reported method [15,16]. After this period of occlusion, the clamps were removed. The animals were randomized into groups receiving different concentrations of rosuvastatin or physiological saline alone by intraperitoneal injection 1 h before ischemia. The maintenance of the animals and the experimental procedures performed on them were carried out in accordance with National Institutes of Health (NIH) guidelines for the use of experimental animals. All procedures were approved by the Animal Care Committee of Kyoto Prefectural University of Medicine (Kyoto, Japan).
The animals were killed after reperfusion, and the intestine was removed and submitted for examination. After making a 30-cm proximal intestinal loop, saline (10 mL) was injected into the loop. The contents were aliquoted after centrifugation. Intestinal injury after reperfusion was evaluated by examining the luminal contents of hemoglobin and protein, and by histological examination. Intestinal bleeding was quantified indirectly as the hemoglobin concentration in luminal lavage fluid using a kit and following the manufacturer’s protocol (Wako). Luminal protein levels were also determined using a kit according to the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA, USA). For histological evaluation, formalin-fixed tissues were stained with hematoxylin and eosin and evaluated by light microscopy by a pathologist who was not informed of the experimental conditions for any given specimen.
The concentrations of TBA-reactive substances were measured in the intestinal mucosa using the method described by Ohkawa et al [17] as an index of lipid peroxidation. The animals were killed by exsanguination from the abdominal aorta after the experiments, and their small intestines were removed. The intestinal mucosa was then scraped off using two glass slides, and homogenized with 1.5 mL of 10 mmol/L potassium phosphate buffer (pH 7.8) containing 30 mmol/L KCl in a Teflon Potter-Elvehjem homogenizer. The level of TBA-reactive substances in the mucosal homogenates was expressed as nanomoles of malondialdehyde per milligram of protein using 1,1,3,3-tetramethoxypropane as the standard. The total protein in the tissue homogenates was measured using a kit, according to the manufacturer’s protocol (Bio-Rad).
Tissue-associated myeloperoxidase (MPO) activity was determined using a modification of the method described by Grisham et al [18] as an index of neutrophil accumulation. Briefly, two milliliters of mucosal homogenates were centrifuged at 20 000 g for 15 min at 4 °C to pellet the insoluble cellular debris. The pellet was then rehomogenized in an equivalent volume of 0.05-M potassium phosphate buffer (pH 5.4), containing 0.5% hexadecyltrimethylammonium bromide. The samples were centrifuged at 20 000 g for 15 min at 4 °C and the supernatants were saved. The MPO activity was assessed by measuring the H2O2-dependent oxidation of 3,3’,5,5’-tetramethylbenzidine. One unit of enzyme activity was defined as the amount of MPO present that caused a change in absorbance of 1.0/min at 655 nm and 25 °C.
Determination of mucosal content of CINC-1 and TNF-α
The concentrations of the inflammatory cytokines TNF-α and CINC-1 in the supernatant of mucosal homogenates were determined by rat TNF-α and CINC-1-specific ELISA kits according to the manufacturer’s instructions.
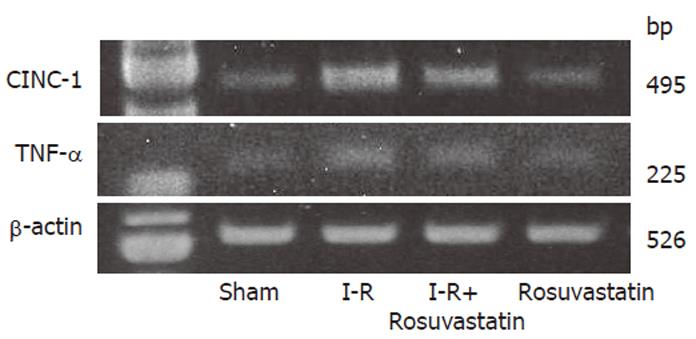
The mRNA expression of intestinal TNF-α and CINC-1 was determined by reverse transciprion-polymerase chain reaction the (RT-PCR). Samples of intestinal tissue for mRNA isolation were removed from the intestine. The total RNA was isolated by the acid guanidinium thiocyanate-phenol-chloroform (AGPC) method using Isogen (Nippon Gene, Tokyo, Japan) and the concentration of RNA was determined by absorbance at 260 nm in relation to that at 280 nm. RNA was stored at –70 °C until it was used for the RT-PCR. Amplification was carried out in a 50-µL mixture containing 2 µL of the RT product, 0.6 µmol/L of both the sense and antisense primers, 0.4 mmol/L dNTP mix, and 0.5 µL Taq DNA polymerase (Takara Shuzo Co., Shiga, Japan). The reaction was performed as follows: 35 cycles of amplication (denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 60 s), followed by a final extension step of 7 min at 72 °C. The primers had the following sequences: for TNF-α, sense 5'-ATGAGCACAGAAAGCATGATC-3', and antisense 5'-TACAGGCTTGTCACTCGAATT-3'; for CINC-1, sense 5’-CTGTGCTGGCCACCAGCCGC-3’, and antisense 5’-ACAGTCCTTGGAACTTCTCTG-3’; and for ß-actin, sense 5’-ATCGTGGGCCGCCCTAGGCA-3’, and antisense 5’-TGGCCTTAGGGTTCAGAGGGG-3’. The PCR products were separated electrophoretically in a 25 g/L agarose gel and stained by ethidium bromide.
Intestinal cells were thawed on ice and homogenized at 4 °C in a solution of 50 mmol/L Tris–HCl (pH 7.6), 300 mmol/L NaCl, 0.5 g/L Triton X-100, 10 g/L aprotinin, 10 g/L leptin, 1 mmol/L phenylmethysulfonyl fluoride (PMSF), 1.8 g/L iodoacetamide, 50 mmol/L NaF, and 1 mmol/L DTT in order to extract the total cell protein. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose (Bio-Rad). Membranes were probed with specific antibodies against eNOS (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and immune complexes were visualized by Western blotting with a commercial kit (ECL, Amersham, Buckinghamshire, England), following the manufacturer’s recommendations.
All results are presented as mean ± SE. The data were compared by two-way analysis of variance (ANOVA), and differences were considered to be significant if the P value was less than 0.05 based on Scheffe’s multiple comparison test. All analyses were performed using the Stat View 5.0-J program (Abacus Concepts, Inc., Berkeley, CA, USA) on a Macintosh computer.
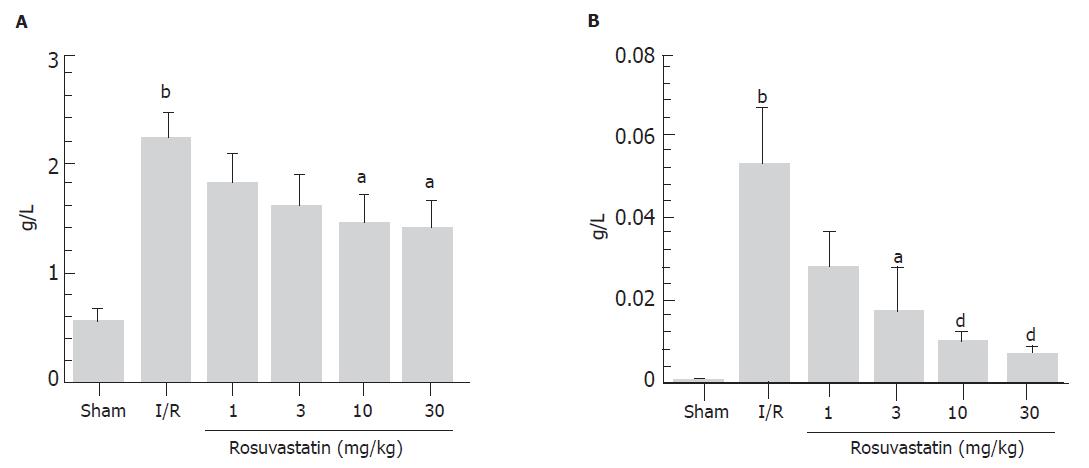
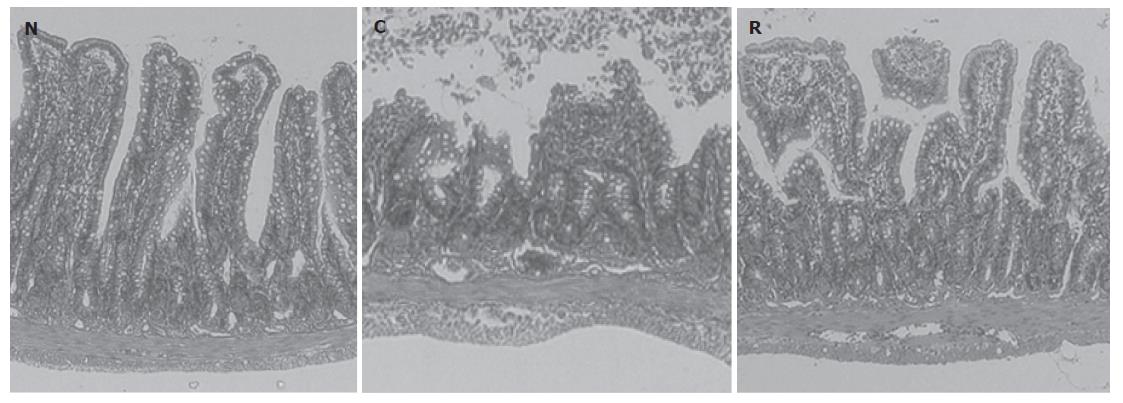
We found that 60 min of reperfusion after 30 min of ischemia resulted in increases in luminal protein and hemoglobin concentrations (Figure 1). In contrast, pre-treatment with rosuvastatin 1 h before the ischemia was found to inhibit these increases in a dose-dependent manner (Figure 1). Neither vehicle alone nor rosuvastatin alone affected the luminal protein or hemoglobin concentrations (data not shown). Therefore, in the following experiments, we used rosuvastatin at a dose of 10 mg/kg and examined its pharmacological action. In rats treated with I-R, multiple erosions and bleeding developed in the small intestine; however, pretreatment with rosuvastatin at a dose of 10 mg/kg 1 h before the ischemia inhibited both the intestinal erosions and the bleeding. The protective effect of rosuvastatin was further confirmed histologically. Representative hematoxylin and eosin-stained sections of the ileum from sham, I-R, and I-R + rosuvastatin-treated animals are depicted in Figure 2. While the ileum of sham animals exhibited normal mucosal architecture with intact villi, I-R resulted in large areas of epithelial crypt loss, predominantly neutrophilic infiltrate throughout the mucosa, erosion, and mucosal bleeding. In contrast, pretreatment with rosuvastatin resulted in smaller erosions with few neutrophils. Rosuvastatin alone did not produce any macroscopic or microscopic lesions in the rat intestine.
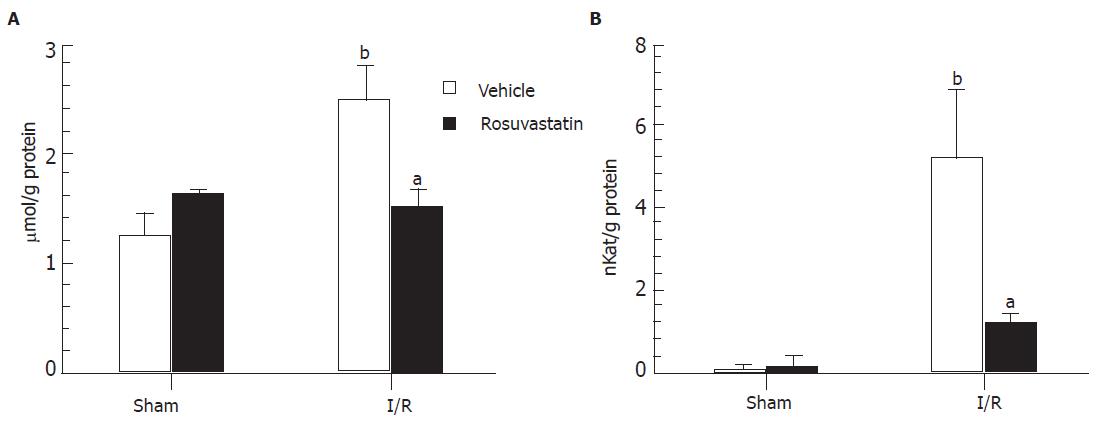
The extent of lipid peroxidation was determined by measuring TBA-reactive substances in the small intestine. No significant differences were found in the intestinal TBA-reactive substance levels between vehicle- and rosuvastatin-treated rats in the sham-operated group. Sixty min of reperfusion caused a significant increase in TBA-reactive substances in the control rats. This increase in TBA-reactive substances in the intestinal mucosa was significantly inhibited by 10 mg/kg of rosuvastatin (Figure 3A).
A hallmark of intestinal reperfusion injury is the accumulation of neutrophils in the injured tissue. Therefore, we next evaluated neutrophil accumulation based on tissue-associated MPO activity. There were no differences in intestinal MPO activities between vehicle- and rosuvastatin-treated rats in the sham-operated group, while the MPO activity in the intestinal mucosa markedly increased from a basal activity of 0.23 ± 0.07 nkat/g protein to 5.20 ± 1.65 nkat/g protein after I-R in the control group. The increase in MPO activity in the intestinal mucosa after reperfusion was significantly inhibited by treatment with rosuvastatin (Figure 3B).
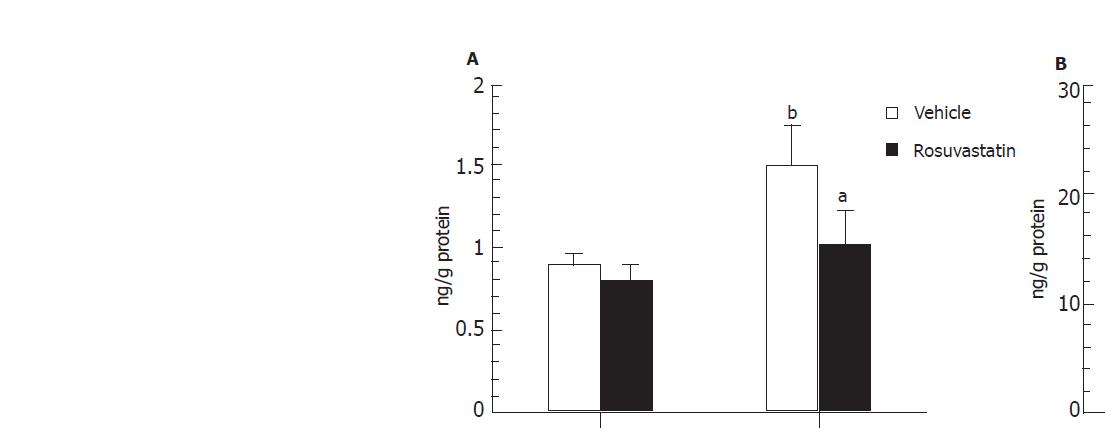
In order to determine whether or not treatment with rosuvastatin can modulate the inflammatory response through the regulation of cytokine production, we analyzed intestinal mucosal levels of CINC-1 and TNF-α. The intestinal concentrations of TNF-α and CINC-1 increased significantly in rats treated with I-R, and these increases were significantly inhibited by treatment with rosuvastatin at a dose of 10 mg/kg (Figures 4A, 4B). To further confirm the inhibitory effect of rosuvastatin on CINC-1 and TNF-α production, we analyzed the intestinal expression of CINC-1 and TNF-α using RT-PCR yielding 495 and 225 base pair products to identify CINC-1 and TNF-α gene expression, respectively. As shown in Figure 5, we found the expression of these genes in rats treated with sham-operation to be negligible or faint. In contrast, transcription was readily enhanced in I-R-treated rats. Treatment with rosuvastatin suppressed the level of mRNA expression for each gene.
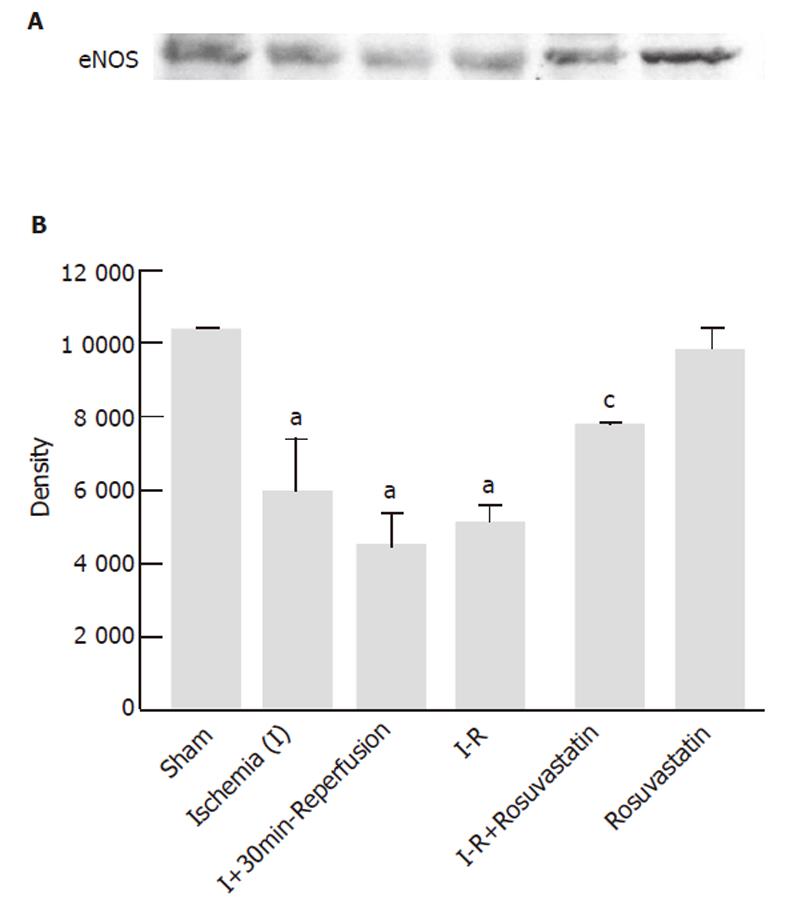
Since previous studies have shown that statins exerts beneficial effects on the endothelium through an increase in NO production from eNOS [19,20], we investigated whether eNOS expression could be affected by I-R with or without rosuvastatin pre-treatment. Figure 6 was a representative of at least 3 experiments performed on different experimental days. The expression levels of eNOS protein were decreased during the I-R. However, pretreatment with rosuvastatin significantly reversed the decrease in eNOS protein. Rosuvastatin alone did not affect intestinal eNOS protein levels in sham-operated rats.
The present study clearly demonstrates that rosuvastatin, a new HMG-CoA reductase inhibitor, has a protective effect against reperfusion-induced intestinal injury and inflammation in rats. In this study, intestinal injury was assessed by a variety of methods including luminal protein/hemoglobin concentrations and histological evaluation. The results of each assessment showed that rosuvastatin treatment significantly inhibited intestinal injury. In addition, we demonstrated that, in I-R-induced intestinal inflammation, the expression of pro-inflammatory cytokines (CINC-1 and TNF-α) was enhanced in association with neutrophil accumulation, as determined by MPO activity in the homogenate of the small intestine. Rosuvastatin treatment inhibited both the up-regulation of pro-inflammatory cytokine expression and the neutrophil infiltration, which prevents tissue injury. These results suggest that rosuvastatin has a protective action against I-R-induced intestinal mucosal injury as well as an anti-inflammatory action against reperfusion-induced inflammation.
Oxygen-derived free radicals produced during I-R are known to cause peroxidation of polyunsaturated fatty acids in cell membranes. The resulting increase in lipid peroxides can lead to changes in membrane fluidity and permeability, and finally to cell lysis. In the present study, TBA-reactive substances in intestinal mucosa, which are an index of lipid peroxidation, were significantly increased in I-R-treated rats compared to sham-operated rats, and rosuvastatin treatment significantly inhibited the increase in these substances. However, neither rosuvastatin nor other statins have been reported to have antioxidant activities in vitro, nor has the inhibition of lipid peroxidation been previously reported. Our recent study also showed that rosuvastatin had no effect on scavenging hydroxyl and superoxide radicals determined by electron spin resonance-spin trapping assay (data not shown). Therefore, the inhibitory effect of rosuvastatin on I-R-induced lipid peroxidation in rat intestinal mucosa may result from its anti-inflammatory activity, rather than from its direct antioxidant activity.
Recent reports hypothesize that neutrophil-mediated inflammation is involved in the pathogenesis of I-R-induced intestinal injury. This hypothesis has been supported by several studies; neutrophil depletion by intraperitoneal injection of anti-neutrophil serum has been found to significantly attenuate intestinal mucosal injury elicited by I-R [21,22]; in addition, immunoneutralization of the CD11/CD18 adherence complex on neutrophils and CINC-1 is known to attenuate intestinal reperfusion injury [23,24]. The present study showed that MPO activity, an index of tissue-associated neutrophil accumulation, increases in the intestinal mucosa after I-R, and that this increase is significantly inhibited by treatment with rosuvastatin. The inhibition of neutrophil-endothelial interaction by statins has been demonstrated in a number of previous reports. Honjo et al [25] have shown that both cerivastatin and pravastatin significantly reduce the number of rolling/accumulated leukocytes in the retinal veins after I-R. Naidu et al [26] have also demonstrated that pretreatment with simvastatin inhibits increases in tissue MPO content in lungs treated with I-R. In addition, the present study showed that the expression of the pro-inflammatory cytokines CINC-1 and TNF-α markedly enhanced in the intestine after I-R, and that these increases are also significantly inhibited by rosuvastatin. The inhibitory effect of statins against mRNA expression for inflammatory genes was also confirmed by a recent study using DNA microarray technology [27], in which it was demonstrated that the statins atorvastatin and pitavastatin directly affect the expression levels of genes involved in inflammation, including interleukin-8 (similar to rat CINC-1), in cultured human umbilical vein endothelial cell. These results, together with the present data, indicate that the inhibition of neutrophil accumulation as well as the inhibition of cytokine production by rosuvastatin may be one of the protective factors decreasing I-R-elicited intestinal mucosal injury.
The beneficial effects of statins on acute inflammation are abolished when it is administered together with an NOS inhibitor [20] or when it was administered to eNOS knockout mice [19], indicating that an enhanced release of NO from eNOS may be involved in the action of statins. Statins are known to up-regulate eNOS expression and activate this enzyme in the systemic vasculature by blocking Rho geranylgeranylation [8,28] as well as by activating the PI-3 kinase/Akt pathway [20]. As shown in the present study, the intestinal levels of eNOS protein gradually decreased during 30-min ischemia and 60-min reperfusion, indicating that reperfusion may be associated with a posttranscriptional reduction in the expression of the eNOS enzyme. In addition, rosuvastatin-treated animals demonstrated preserved expression of eNOS protein compared to the I-R-treated group, although this agent did not affect the level of eNOS protein in normal rats. Therefore, the persistent expression of eNOS protein at 60 min of reperfusion in rosuvastatin-treated rats is likely to explain the reduction in tissue injury and neutrophil accumulation. In line with our observations, NO is reported to prevent leukocyte adhesion to the endothelium by repressing the up-regulation of cell adhesion molecules in the endothelial cells [29,30].
Our results showed that I-R-induced intestinal inflammation is characterized by increased production of inflammatory mediators and decreased expression of eNOS. The blockade of these mediators and the preservation of eNOS expression by rosuvastatin were accompanied by significant suppression of intestinal inflammation in vivo. These data suggest that rosuvastatin may represent a novel therapeutic approach for the treatment of I-R-induced intestinal injury.
| 1. | Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am. 1998;27:827-860, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg. 1997;14:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699-H703. [PubMed] |
| 4. | Palluy O, Morliere L, Gris JC, Bonne C, Modat G. Hypoxia/reoxygenation stimulates endothelium to promote neutrophil adhesion. Free Radic Biol Med. 1992;13:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Yoshida N, Granger DN, Anderson DC, Rothlein R, Lane C, Kvietys PR. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992;262:H1891-H1898. [PubMed] |
| 6. | Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 851] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Oda H, Keane WF. Recent advances in statins and the kidney. Kidney Int Suppl. 1999;71:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35:2708-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 747] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Ikeda Y, Young LH, Lefer AM. Rosuvastatin, a new HMG-CoA reductase inhibitor, protects ischemic reperfused myocardium in normocholesterolemic rats. J Cardiovasc Pharmacol. 2003;41:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Bulhak A, Sjoquist PO, Pernow J. Rosuvastatin protects the myocardium against ischaemia-reperfusion injury via inhibition of GGPP synthesis. Cardiovasc J S Afr. 2004;15:S11. |
| 12. | Weinberg EO, Scherrer-Crosbie M, Picard MH, Nasseri BA, MacGillivray C, Gannon J, Lian Q, Bloch KD, Lee RT. Rosuvastatin reduces experimental left ventricular infarct size after ischemia-reperfusion injury but not total coronary occlusion. Am J Physiol Heart Circ Physiol. 2005;288:H1802-H1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Bulhak AA, Gourine AV, Gonon AT, Sjöquist PO, Valen G, Pernow J. Oral pre-treatment with rosuvastatin protects porcine myocardium from ischaemia/reperfusion injury via a mechanism related to nitric oxide but not to serum cholesterol level. Acta Physiol Scand. 2005;183:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Di Napoli P, Taccardi AA, Grilli A, De Lutiis MA, Barsotti A, Felaco M, De Caterina R. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc Res. 2005;66:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Naito Y, Takagi T, Uchiyama K, Handa O, Tomatsuri N, Imamoto E, Kokura S, Ichikawa H, Yoshida N, Yoshikawa T. Suppression of intestinal ischemia-reperfusion injury by a specific peroxisome proliferator-activated receptor-gamma ligand, pioglitazone, in rats. Redox Rep. 2002;7:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Naito Y, Takagi T, Ichikawa H, Tomatsuri N, Kuroda M, Isozaki Y, Katada K, Uchiyama K, Kokura S, Yoshida N. A novel potent inhibitor of inducible nitric oxide inhibitor, ONO-1714, reduces intestinal ischemia-reperfusion injury in rats. Nitric Oxide. 2004;10:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 19226] [Article Influence: 409.1] [Reference Citation Analysis (1)] |
| 18. | Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567-G574. [PubMed] |
| 19. | Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880-8885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 752] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 20. | Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, Klein HH, Dominiak P, Richardt G. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol. 2004;44:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269-H1275. [PubMed] |
| 22. | Kurtel H, Fujimoto K, Zimmerman BJ, Granger DN, Tso P. Ischemia-reperfusion-induced mucosal dysfunction: role of neutrophils. Am J Physiol. 1991;261:G490-G496. [PubMed] |
| 23. | Slocum MM, Granger DN. Early mucosal and microvascular changes in feline intestinal transplants. Gastroenterology. 1993;105:1761-1768. [PubMed] |
| 24. | Yagihashi A, Tsuruma T, Tarumi K, Kameshima T, Yajima T, Yanai Y, Watanabe N, Hirata K. Prevention of small intestinal ischemia-reperfusion injury in rat by anti-cytokine-induced neutrophil chemoattractant monoclonal antibody. J Surg Res. 1998;78:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Honjo M, Tanihara H, Nishijima K, Kiryu J, Honda Y, Yue BY, Sawamura T. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol. 2002;120:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga C, Mulligan MS. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2003;126:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Morikawa S, Takabe W, Mataki C, Kanke T, Itoh T, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb. 2002;9:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1232] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 29. | Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 2102] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 30. | Lefer AM. Nitric oxide: nature's naturally occurring leukocyte inhibitor. Circulation. 1997;95:553-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
S- Editor Guo SY L-Editor Zhang JZ E- Editor Zhang Y