Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1881
Revised: October 2, 2005
Accepted: October 26, 2005
Published online: March 28, 2006
AIM: To develop the effective technology for reconstruction of a liver organ in vitro using a bio-artificial liver.
METHODS: We previously reported that a radial-flow bioreactor (RFB) could provide a three-dimensional high-density culture system. We presently reconstructed the liver organoid using a functional human hepatocellular carcinoma cell line (FLC-5) as hepatocytes together with mouse immortalized sinusoidal endothelial cell (SEC) line M1 and mouse immortalized hepatic stellate cell (HSC) line A7 as non parenchymal cells in the RFB. Two x 107 FLC-5 cells were incubated in the RFB. After 5 d, 2 x 107 A7 cells were added in a similar manner followed by another addition of 107 M1 cells 5 d later. After three days of perfusion, some cellulose beads with the adherent cells were harvested. The last incubation period included perfusion with 200 nmol/L swinholide A for 2 h and then the remaining cellulose beads along with adherent cells were harvested from the RFB. The cell morphology was observed by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). To assess hepatocyte function, we compared mRNA expression for urea cycle enzymes as well as albumin synthesis by FLC-5 in monolayer cultures compared to those of single-type cultures and cocultures in the RFB.
RESULTS: By transmission electron microscopy, FLC-5, M1, and A7 were arranged in relation to the perfusion side in a liver-like organization. Structures resembling bile canaliculi were seen between FCL-5 cells. Scanning electron microscopy demonstrated fenestrae on SEC surfaces. The number of vesiculo-vacuolar organelles (VVO) and fenestrae increased when we introduced the actin-binding agent swinholide-A in the RFB for 2h. With respect to liver function, urea was found in the medium, and expression of mRNAs encoding arginosuccinate synthetase and arginase increased when the three cell types were cocultured in the RFB. However, albumin synthesis decreased.
CONCLUSION: Co-culture in the RFB system can dramatically change the structure and function of all cell types, including the functional characteristics of hepatocytes. Our system proves effective for reconstruction of a liver organoid using a bio-artificial liver.
- Citation: Saito M, Matsuura T, Masaki T, Maehashi H, Shimizu K, Hataba Y, Iwahori T, Suzuki T, Braet F. Reconstruction of liver organoid using a bioreactor. World J Gastroenterol 2006; 12(12): 1881-1888
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1881
Liver regeneration technology has made many advances in recent years. Efforts now are being made toward development of embryonic stem cells (ES cells), differentiation of hemopoietic stem cells, and development of isolation and culture methods for somatic stem cells originating from different organs. Hemopoietic stem cells, hepatoblasts originating from fetal liver, hepatocytes, and pancreatic duct epithelial cells have been included in the list of candidate cells for liver regeneration[1]. Development of immortalized cells by introduction of the simian virus 40 (SV40) large T antigen gene or human telomerase reverse transcriptase (hTERT) is also under investigation[2]. To date, however, no technique for regenerating and reconstructing parenchymal organs using these cells has been established. Conventional cell culture methods have achieved this goal clinically for skin, cornea, and bone tissue[3,4].
Reconstruction of organs such as the liver requires maintenance of viable cells at a high density and coculture under conditions favorable to several different cell types that constitute a liver. To make a culture system is important in reconstructing a liver organoid. Conventional stationary culture techniques are not well suited to the culture of cells in a layered form, i.e., in a structural and functional organoid a simple air/CO2 incubator does not deliver adequate oxygen supply to layered cells. Furthermore, high-density culture cannot be maintained with the limited nutrients available in conventional cultures. For these reasons, construction of a bioreactor that allows 3-dimensional growth in a high-density perfusion culture has been advocated for reconstructing a liver organoid. In our study a radial-flow bioreactor (RFB) developed in Japan was used as a candidate model for high-density perfusion culture. Filled with a porous carrier, this bioreactor permits culture at a cell density 10 times higher than that allowed by a hollow-fiber culture system[5,6]. Another important point is to select a cell source. Clinically, cells using bio-artificial liver are required to be highly functional and supplied quickly in large quantities. Therefore we established a functional human hepatocellular carcinoma cell line (FLC-5), which can express drug-metabolized enzymes (e.g., human-type carboxyl esterase or cytochrome) and liver-specific proteins such as albumin. In vitro this cell line retains its three-dimensional form, developing distinct microvilli on the surface. These cells can be cultured in serum-free ASF104 medium (Ajinomoto, Tokyo). A liver organoid cannot be reconstructed with hepatocytes only. At minimum, coculture of hepatocytes with nonparenchymal cells, such as sinusoidal endothelial cell (SEC) and hepatic stellate cell (HSC) is required. So we established immortalized SEC line M1[7] and an immortalized HSC line A7[8] by isolating nonparenchymal cells from an H-2Kb-tsA58-transgenic mouse liver transfected with the SV40 large T antigen gene[9].
Reconstruction of the liver sinusoid is important for activity of the liver organoid as a functional unit. Also, the open pores on the surfaces of SEC in fenestrae have an important functional role in the liver sinusoid. Fenestrae are the most remarkable characteristics of SEC, as first described by Wisse in 1970[10] using transmission electron microscopy (TEM). Diameters of these pores vary between species, ranging from 100 to 200 nm[11]. These fenestrae facilitate the transport of materials and solutes from the luminal to the abluminal side of the liver parenchymal cells and vice versa[12]. The process and mechanism of formation of these pores remain largely unclarified[13,14]. The presence of actin filaments at the margin of these pores has been demonstrated by electron microscopic studies[15,16]. Swinholide A, a most potent microfilament-disrupting drug available, has been demonstrated to increase the number of SEC fenestrae[13]. However, when immortalized SEC was treated in a monolayer culture or as a monoculture in the RFB, an increase in number of fenestrae could not be observed when the Swinholide A was introduced. The potential for drug-induced increase also has been reported to disappear in long-term cultures[7].
In developing a high functioning organoid using a bio-artificial liver, the function, form and reactivity of pharmacological agent should be near in vivo. In the present study, we reconstructed a functional liver organoid using immortalized cell lines in the RFB.
We used the three cell lines mentioned above, FLC-5, M1, and A7. As reported, culture of M1 cells was possible in serum-free conditions while supplementation of ASF104 medium with 2% fetal bovine serum (FBS) was required for culture of A7 cells. Therefore, in coculture experiments, ASF104 medium was enriched with 2% FBS.
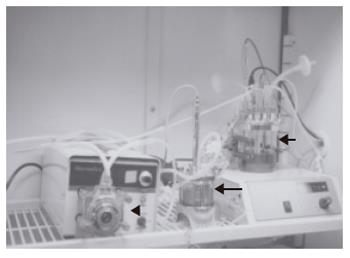
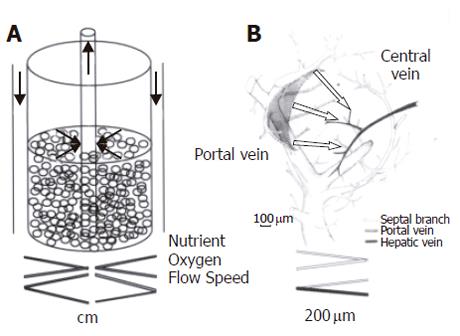
As reported elsewhere, the RFB system is composed of a 15-mL radial-flow chamber (RA-15; ABLE, Tokyo), a mass flow controller (RAD925, ABLE), a reservoir (Figure 1), a computer, and a tissue incubator as described previously[17] (Figure 1). The culture medium was oxygenated within the reservoir, and the pH was adjusted automatically to 7.4. Oxygen pressure in the culture medium was measured both within the reservoir and at the outlet of the bioreactor. Relative oxygen consumption was monitored on the basis of the oxygen pressure gradient. During the study the temperature within the reservoir was kept constantly at 37 °C. Two × 107 FLC-5 cells were inoculated into the reservoir. The bioreactor was perfused in a closed circuit for 2 h to aid cells in adhering to the porous carrier cellulose beads (Asahi Kasei, Tokyo). Subsequently the bioreactor was switched to the open-circuit mode, and incubation was continued with addition of fresh culture medium to the reservoir. After 5 d, 2 ×107 A7 cells were added in a similar manner followed by another addition of 107 M1 cells 5 d later. Retinol (10-6mol/L) was added during the first 2 d. After three days of perfusion cellulose beads with the adherent cells were harvested, and cells deposited at the bottom of the bioreactor also were recovered. Beads with attached cells were fixed in 1.2% or 2.0% glutaraldehyde as described below.
We cultured the three cell lines as described above. The last incubation included perfusion with 200 nmol/L swinholide A (Sigma catalog number S9810; S) for 2 h. The cellulose beads along with adherent cells were harvested from the bioreactor. Beads with attached cells were fixed in glutaraldehyde and prepared for morphologic observation as follows.
For scanning electron microscopy (SEM), cultured cells were fixed with 1.2% glutaraldehyde in 0.1 mol/L phosphate buffer (PB) at pH 7.4 and postfixed with 1% OsO4 in 0.1mol/L PB. The fixed cells were rinsed twice with PBS, subsequently dehydrated in ascending concentrations of ethanol, critical point-dried using carbon dioxide, and coated by vacuum- evaporated carbon and ion-spattered gold. Specimens were observed under JSM-35 scanning electron microscope (JEOL, Tokyo) at an accelerated voltage of 10 kV.
For transmission electron microscopy (TEM), cultured cells were fixed with 2.0% glutaraldehyde in 0.1mol/L PB for 1 h and postfixed with 1% OsO4 in 0.1mol/L PB for 1 h at 4 °C. Specimens were dehydrated in ethanol and subsequently embedded in a mixture of Epon-Araldite. Thin sections (60 nm) were cut with a diamond knife mounted on an LKB ultratome, and stained with aqueous uranyl acetate. Specimens were examined under a JEOL 1200EX electron microscope.
For analysis of amino acid fractions by high-performance liquid chromatography (HPLC), supernatants were collected from FLC-5 alone and from cocultures of the three cell types in the bioreactor. Supernatants were mixed with 5% sulfosalicylic acid and allowed to stand at 4 °C for 15 min. After centrifugation to precipitate protein, supernatants were injected into amino acid analysis columns (L-8500, Hitachi, Tokyo).
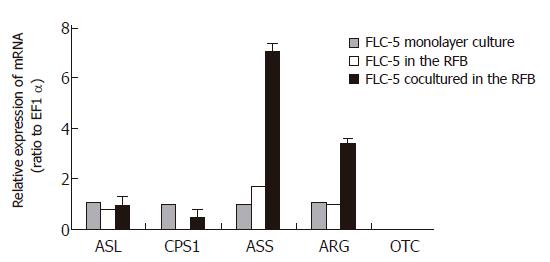
We measured mRNA expression for the urea cycle enzymes, carbamoyl phosphate synthetase (CPS1), ornithine carbamoyltransferase (OCT), argininosuccinatesynthetase (ASS), argininosuccinatelyase (ASL), and arginase (ARG), as well as mRNA expression for albumin, hepatocyte nuclear factor (HNF)-1 and HNF-4, by quantitative TaqMan reverse transcription polymerase chain reaction (RT-PCR). RT-PCR was performed on the ABI PRISM 7700 sequence detection system using random hexamers from TaqMan reverse transcription reagents and the RT reaction mix (Applied Biosystems, Rockville, M) to reverse-transcribe RNA. TaqMan universal PCR Master Mix and Assays-on-Demand gene expression probes (Applied Biosystems) were used for PCR. A standard curve for serial dilution of 18S rRNAs was generated similarly. A relative standard curve method (Applied Biosystems) was used to calculate the amplification difference in urea cycle-related enzymes between cocultured and control cells, and elongation factor 1 (EF1), for each primer set and between albumin, HNF-1, HNF-4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Specificity was evaluated using GAPDH mRNA as an internal control (4310884E; Perkin-Elmer Applied Biosystems). Each amplification was performed in triplicate, and averages were obtained.
Based on DNA sequences in GenBank, primers and the TaqMan probe for albumin, HNF-1, and HNF-4 were designed using the primer design software Primer Express TM (Perkin-Elmer Applied Biosystems, Foster City, CA). AmpliTaq DNA polymerase extended the primer and displaced the TaqMan probe through its 5’-3’ exonuclease activity. Probes were labeled with a reporter fluorescent dye either 6-carboxy-fluorescein (FAM) or 2, 7 dimethoxy-4,5-dichloro-6-carboxy-fluorescein (JOE) at the 5’ end and a quencher fluorescent dye [6-carboxytetramethyl-rhodamine (TAMRA)] at the 3’ end.
Primers/probes were as follows: ornithine transcarbamoylase (OTC) forward primer 5’-CCAGGCAATAAAAGAGTCAGGATT-3’, reverse primer/ 5’-TTATCAAAG TCCCCTGGTTAGAGATACT-3’, probe/ 5’-(FAM)- TTCAAATGCTCCTACACCCTGCCCTG-(TAMRA)-3’; arginosuccinase (ASL) forward primer/ 5’-TGGCCAAGGAGGTCGTCA-3’, reverse primer 5’-TTCCTCGTCGTCCGGAAG-3’, probe 5’-(FAM)-TGTCTTCCAGACCCGGAGACCGAA-(TAMRA)-3’; albumin forward primer/ 5’-CGATTTTCTTTTTAGGGCAGTAGC-3’, reverse primer/ 5’-TGGAAACTTCTGCAAACTCAGC-3’, probe/ 5’-(FAM)-CGCCTGAGCCAGAGATTTCCCA-(TAMRA)-3’; HNF-1 forward primer/ 5’-AGCGGGAGGTGGTCGATAC-3’, reverse primer/ 5’-CATGGGAGTGCCCTTGTTG-3’, probe/ 5’-(FAM)-TCAACCAGTCCCACCTGTCCCAACA-(TAMRA)-3’; HNF-4 forward primer/ 5’-GGTGTCCATACGCATCCTTGA-3’, reverse primer/ 5’-TGGCTTTGAGGTAGGCATACTCA-3’, probe/ 5’-(FAM)-CCTTCCAGGAGCTGCAGATCGATGAC-(TAMRA)-3’; GAPDH forward primer/ 5’-CTCCCCACACACATGCACTTA-3’, reverse primer/ 5’-CCTAGTCCCAGGGCTTTGATT-3’, probe/ 5’-(VIC)-AAAAGAGCTAGGAAGGACAGGCAACTTGGC- (TAMRA)-3’.
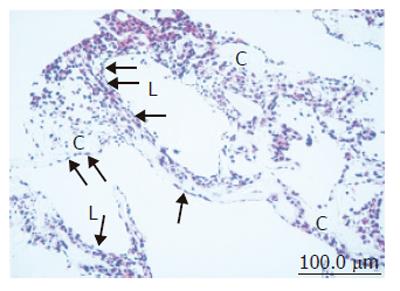
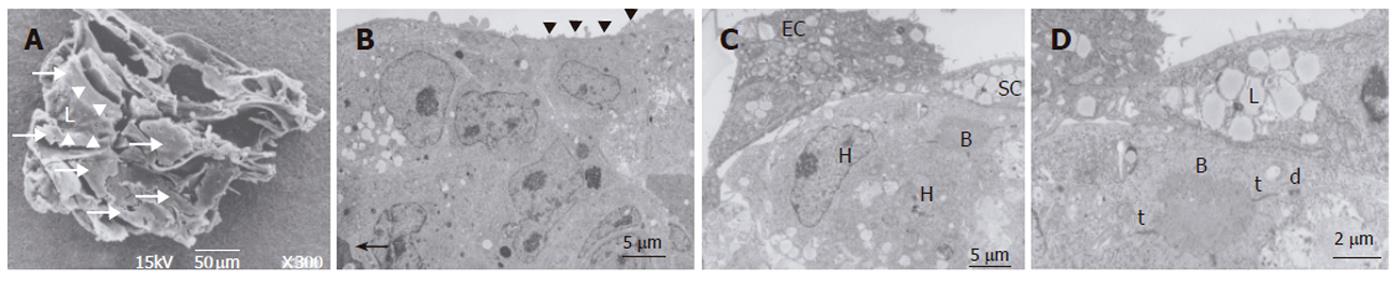
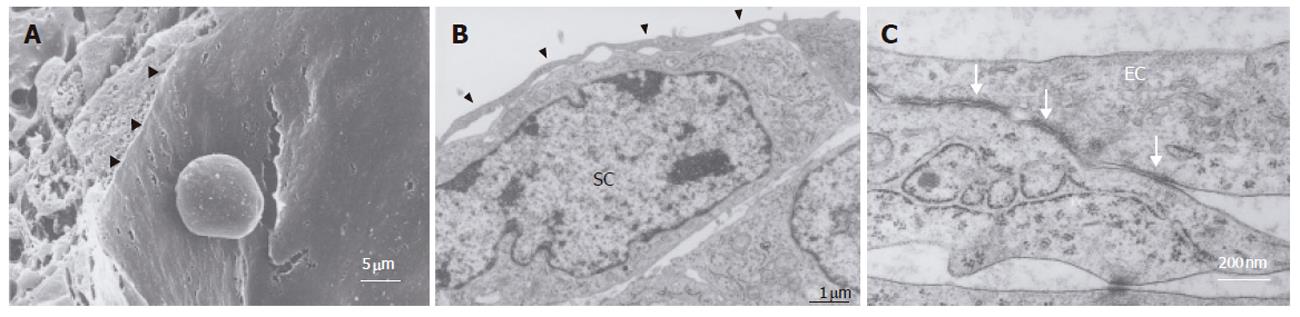
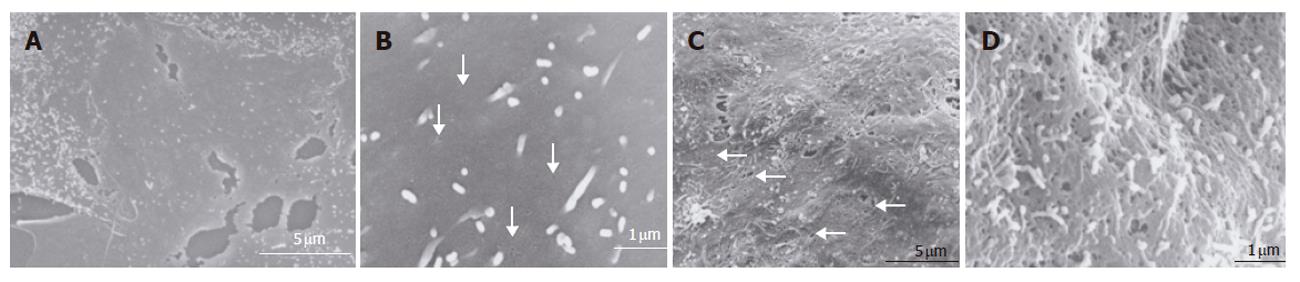
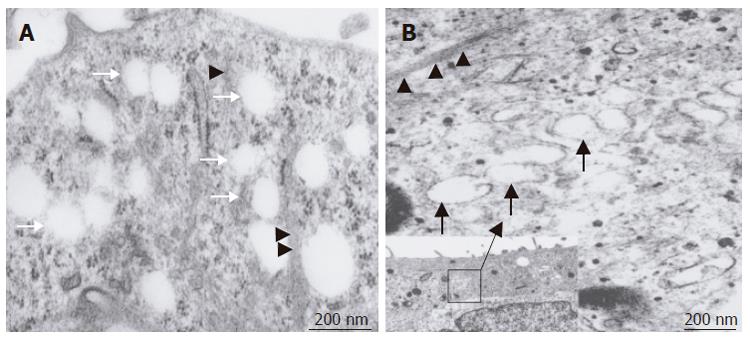
In the bioreactor, cells cultured in high density assumed layered form on the cellulose beads. Lumen-like structure was observed. Endothelial cells were exits with flat shape at the surface of the lumen and the perfusion side (Figure 2). Multiple layers of FLC-5 cells adhered to the cellulose beads, while A7 and M1 cells were predominantly localized to the side where perfusion occurred. Layered cells were seen in a hole of porous cellulose beads. Sinusoid-like lumen was observed at perfusion side in the cellulose beads (Figure 3A). TEM showed that cocultured cells assumed layered form from cellulose beads to the perfusion side (Figure 3B). M1 and A7 cells containing vitamin A-laden fat droplets were seen mainly at the perfusion side, while dense layers of FLC-5 cells were observed beneath (Figure 3C). At sites where the three cell lines were in contact with each other bile canaliculus-like structures were present between neighboring FLC-5 cells. Lumens of these structures contained electron-dense bile components, tight junctions and desmosomes also could be observed (Figure 3D). This side showed growth of endothelial cells with the formation of sinusoid-like vascular structures (Figures 4A and 4B). Tight junctions were seen between endothelial cells (Figure 4C). Fenestrae which are characteristic of SEC in vivo, were absent in monocultures of M1 cells on plastic dishes (Figures 5A and 5B). Because a long time subculture would change the character of M1 cells, pores were represent on the surface of M1 cells co-cultured in the RFB system (Figure 5C). The pores had a diameter of 100 to 200 nm, being similar in morphology and size to those of fenestrae shown by SEC in vivo (Figure 5D).
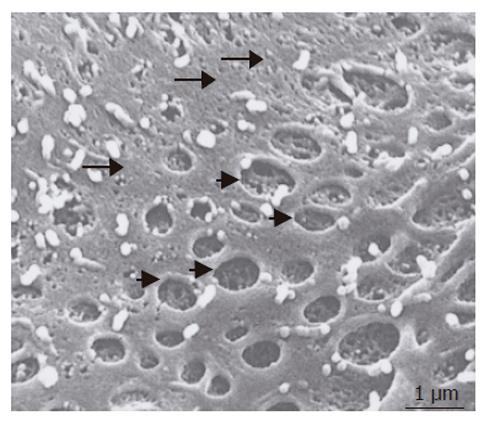
Cells incubated for 2 h with 200 nmol/L of the actin-disrupting agent swinholide A showed the increased number of pores (Figure 6), while some pores were dilated (about 1 μm). Small pores (tens of nanometers in size) that probably resembled coated pits were abundant in the nonfenestrated areas.
TEM investigation showed that treatment with swinholide A resulted in fenestrated pores with a diameter between 100 and 200 nm. The pores fused with each other formed labyrinthine structures (Figure 7A). In addition, vacuoles with a diameter of about 200 nm, similar to previously described vesiculo vacuolar organelles (VVO), were noted. These structures typically were seen in areas where relatively regular overlap was seen in FLC-5, A7, and M1. The number of VVO increased when cells were treated with 200 nmol/L swinholide A, which was associated with partial fusion (Figure 7B).
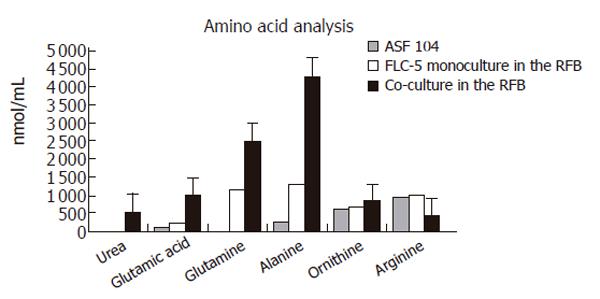
At the end of culture, the supernatant was subjected to amino acid analysis. Urea production was not seen in monocultures of FLC-5 cells in the RFB, while FLC-5 cells cocultured with M1 and A7 cells produced 523 nmol urea /mL in the culture medium, suggesting that the urea cycle was activated in the coculture RFB system (Figure 8). Several amino acids were increased in the medium.
We compared mRNA expression of CPS1, OTC, ASS, ASL, and ARG in FLC-5 monolayer cultures with those of monocultures in the RFB system. In addition, mRNA expression in cocultures in the RFB also was assessed. We could not detect OTC in any type of culture. Expression of other urea cycle enzymes showed no notable difference between monolayer culture of FLC-5 and monoculture of FLC-5 in the RFB. However, ASS and ARG expressions in co-culture in the RFB were about 7 and 3 times greater than those in FLC-5 monolayer culture (Figure 9).
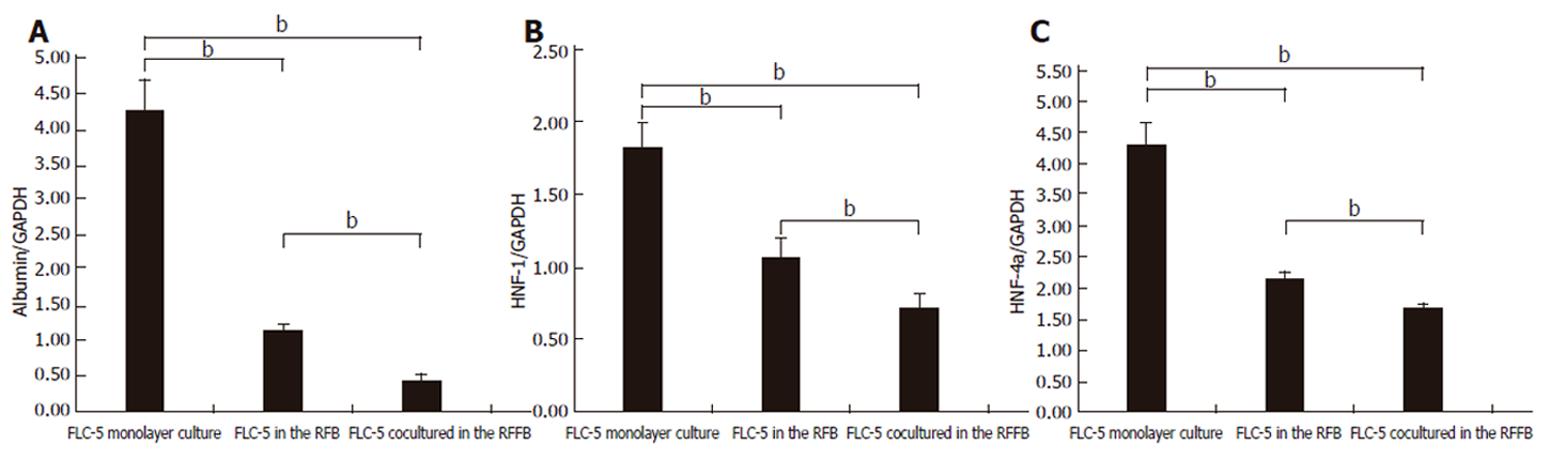
We compared mRNA expression of albumin and HNF-1 and HNF-4 as transcription regulation factors between experimental conditions. Expression of mRNA encoding the three proteins was less in FLC-5 co-cultures in the RFB system than in FLC-5 RFB monocultures or in FLC-5 cells in monolayer culture (Figures 10A-10C). In a previous study, albumin production was enhanced in the RFB using the immortalized cell line[17]. However it was different cell line in this study.
Introduction of a functional human hepatocellular carcinoma cell line (FLC) in our system can allow the cells to be cultured at high density in a layered array and maintain viability for long periods[17,18].
Immortalized cells can be used for artificial liver. The reason is that it can supply cells in large quantities and quickly. Immortalized cells lose several characteristics in morphology and function. However, three cell lines were studied in our RFB culture system, including their fine structure according to electron microscopy. Layers of FLC-5, A7, and M1 were arranged respectively from the carrier attachment side to the perfusion side. In some areas, liver-like architectures, sinusoid-like lumen structure, bile-canaliculi and functional complex, were observed, comparable to in vivo tissue relationship. The M1 cell line well covered the perfusion side, mimicking vascular structures, indicating that this cell type forms an arrangement similar to that in vivo. Furthermore, M1 cells in monolayer culture did not express fenestrae, probably reflecting a long culture or subculture time[19]. In a previous study, we found that M1 cells also lack fenestrae in monocultures in the RFB[7]. In contrasts, fenestrated pores were seen in M1 cells cocultured according to the present RFB experimental design. Coculture and cell to cell contact have an influence on these morphological changes. Because fine structures in vivo could be observed better than monoculture in the RFB.
The electron microscopic observations in the present study clearly showed that if an appropriate environment for cell growth was provided in a perfusion culture system, the individual cell types could arrange themselves according to their in vivo characteristics, even in a high-density layered culture.
This study also examined the numerical dynamics of fenestrae. For this we exposed the cocultures to the actin-disrupting drug swinholide A[13]. When the cells were treated with swinholide A, the number of pores with a diameter of about 100 to 200 nm increased 2 h after swinholide A treatment. Furthermore, by TEM, cytoplasmic vesicles about 200 nm in diameter could be seen and were much larger than the caveolae in the cytoplasm, and their number increased in the presence of swinholide A. These vacuolar-like vesicles probably represent the vesiculo vacuolar organelle (VVO) as described by Feng et al[20]. The VVO is an organelle contributing to transport of macromolecules between luminal and abluminal sides of endothelial cells, thus increasing transcellular permeability. Vascular permeability factor and vascular endothelial growth factor (VPF/VEGF) can induce formation of VVO[21]. FLC-5 used in this study, could express VPF/VEGF (data not shown). The presence of vascular factors may partially explain why VVO is noted in cocultures and why fenestrae could be observed in our experiments[22]. VVO is thought to be formed by fusion of caveolae, when multiple VVOs fuse together, a structure extending from the luminal to the abluminal sides of endothelial cells is formed. In the present study, fused VVOs also were seen in swinholide A - treated specimens by transmission electron microscopy, suggesting that this fusion represents a process culminating in formation of the labyrinthine structures in SEC[23]. The mechanism of pore formation in immortalized SEC and under cocultured perfusion conditions remains unknown from the present study. However, pore formation may result from multiple effects or factors working in concert upon endothelial cells, such as cytoskeletal dynamics represented by actin and/or the influence of a yet unknown factor secreted by other cell types present in the cocultures such as VEGF. The observation that hepatic endothelial cells maintain one of their typical morphological features (i.e. an abundant number of membrane-bound coated-pits, uncoated vesicles/vacuoles and fenestrae) is an indication that the bioreactor mimics a nearby physiological cultivation environment for the various liver cell types. However, the mechanism by which the bioreactor and its culture environment bring about and maintain these membrane-bound vesicles and fenestrae in endothelial cells remain to be elucidated and consequently open up new directions for future experiments.
To assess hepatocyte function, we compared mRNA expression for urea cycle enzymes and albumin synthesis by FLC-5 in monolayer culture compared to these single-type cultures and cocultures in the RFB. Previously, we have demonstrated hepatocyte functions such as albumin synthesis and cytochrome expression are enhanced in the RFB[24,25]. Urea production is among the most primitive functions of liver cells. We could not detect urea in medium from monolayer cultures or monocultured FLC-5 in the RFB. In contrast, FLC-5 cells cocultured in the RFB exhibit ability to produce urea, and mRNA expression for ASS and ARG is enhanced. The medium used in this experiment, ASF 104 contained arginine, so urea production was observed in cocultures in the RFB although OTC was not expressed. One report showed that urea production in OTC-deficient mice could be detected under the same condition[26]. Glutamic acid, glutamine and alanine were also increased in supernatant co-cultured in the RFB, indicating that amino acid metabolism becomes active.
It was reported that three-dimensional spherical culture induces albumin synthesis, a particularly important hepatocytic function[27,28]. However, in the present study, mRNA expression of albumin was decreased under co-culture conditions in the RFB. Nuclear transcriptional factors HNF-4 and HNF-1, which regulate albumin synthesis, were decreased under coculture conditions in the RFB. Albumin in supernatant was also decreased during culture (data not shown). The results suggest that the culture environment (cell-to-cell communication, cell polarity, shear stress, and other factors) can control manifestations of intracellular nuclear transcription factors and therefore dramatically influence albumin production by liver cells. Immortalized cells can be used for artificial liver. The reason is that it can supply cells in large quantities and quickly. But immortalized cells may change the characteristics of its original cells. In this study, albumin synthesis was decreased. It was not useful for artificial liver. In future study, we have to try other cell sources (ES cell, oval cell, and other immortalized cell lines).
Finally, several points should be noted concerning our culture system. First, controlling the mixture ratio of the three cell types used is very difficult since each type possesses its own potential for active growth. Thus, growth rates vary between cell types and are difficult to control. For examples, A7 cells grew less rapidly and tended to be less than the other two cell types in the coculture system. Second, the hepatic lobule spans about 140 μm in vivo, extending from the portal to the central area, toward which portal blood flows in a radial manner. According to Matsumoto et al[29], the liver is an organ composed of numerous groups of microscopic three-dimensional units (minimal radial-flow bioreactors) extending from the inflow side (composed of combinations of parabola-shaped inflow fronts) to the central vein[30]. According to this model, the liver microcirculation as observed in vivo could not be reproduced faithfully with a radial-flow bioreactor, since the distance between inflow and outflow sides in the bioreactor is about 1.5 cm (Figure 11). Third, bile canaliculus-like structures are formed between hepatocytes. Since we did not use the bile duct cells in this study, whether different cell types can reconstruct bile ducts remains to be elucidated[31]. Finally, although several questions remain, the results of the present study suggest that liver reconstruction is possible in vitro. Such organ reconstruction technology is expected to contribute greatly to the development of sophisticated artificial livers and other organs for transplantation. Our culture system may be a very important tool to maintain liver organ.
The authors thank Mr. Hideki Saito, Mrs. Emi Kikuchi, and Mrs. Hisako Arai of the DNA Medical Institute at The Jikei University School of Medicine for technical assistance with electron microscopy. The authors also thank the members of the Australian Key Center for Microscopy and Microanalysis of The University of Sydney for their excellent administrative, technical, and practical support.
| 1. | Gordon GJ, Butz GM, Grisham JW, Coleman WB. Isolation, short-term culture, and transplantation of small hepatocyte-like progenitor cells from retrorsine-exposed rats. Transplantation. 2002;73:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Kanda T, Watanabe S, Yoshiike K. Immortalization of primary rat cells by human papillomavirus type 16 subgenomic DNA fragments controlled by the SV40 promoter. Virology. 1988;165:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1053] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 4. | Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9 Suppl 1:S5-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Sussman NL, Chong MG, Koussayer T, He DE, Shang TA, Whisennand HH, Kelly JH. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology. 1992;16:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 215] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Sussman NL, Kelly JH. Improved liver function following treatment with an extracorporeal liver assist device. Artif Organs. 1993;17:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Matsuura T, Kawada M, Hasumura S, Nagamori S, Obata T, Yamaguchi M, Hataba Y, Tanaka H, Shimizu H, Unemura Y. High density culture of immortalized liver endothelial cells in the radial-flow bioreactor in the development of an artificial liver. Int J Artif Organs. 1998;21:229-234. [PubMed] |
| 8. | Matsuura T, Kawada M, Sujino H, Hasumura S, Nagamori S, Shimizu H. Vitamin A metabolism of immortalized hepatic stellate cell in the bioreactor. Cells of the Hepatic Sinusoid 7. Leiden: Kupffer Cell Foundation 1999; 88-89. |
| 9. | Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096-5100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 607] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 427] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Braet F. How molecular microscopy revealed new insights into the dynamics of hepatic endothelial fenestrae in the past decade. Liver Int. 2004;24:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863-874. [PubMed] |
| 13. | Braet F, Spector I, De Zanger R, Wisse E. A novel structure involved in the formation of liver endothelial cell fenestrae revealed by using the actin inhibitor misakinolide. Proc Natl Acad Sci U S A. 1998;95:13635-13640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Braet F, Spector I, Shochet N, Crews P, Higa T, Menu E, de Zanger R, Wisse E. The new anti-actin agent dihydrohalichondramide reveals fenestrae-forming centers in hepatic endothelial cells. BMC Cell Biol. 2002;3:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Oda M, Tsukada N, Komatsu H, Kaneko K, Nakamura M, Tsuchiya M. Electron microscopic localizations of actin, calmodulin and calcium in the hepatic sinusoidal endothelium in the rat. In: Kirn A, Knook DL, Wisse E des. Cells of the Hepatic Sinusoid 1. Rijswijk, Kupffer Cell Foundation. 1986;511-512. |
| 16. | Gatmaitan Z, Varticovski L, Ling L, Mikkelsen R, Steffan AM, Arias IM. Studies on fenestral contraction in rat liver endothelial cells in culture. Am J Pathol. 1996;148:2027-2041. [PubMed] |
| 17. | Kawada M, Nagamori S, Aizaki H, Fukaya K, Niiya M, Matsuura T, Sujino H, Hasumura S, Yashida H, Mizutani S. Massive culture of human liver cancer cells in a newly developed radial flow bioreactor system: ultrafine structure of functionally enhanced hepatocarcinoma cell lines. In Vitro Cell Dev Biol Anim. 1998;34:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Aizaki H, Nagamori S, Matsuda M, Kawakami H, Hashimoto O, Ishiko H, Kawada M, Matsuura T, Hasumura S, Matsuura Y. Production and release of infectious hepatitis C virus from human liver cell cultures in the three-dimensional radial-flow bioreactor. Virology. 2003;314:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Braet F, de Zanger R, Seynaeve C, Baekeland M, Wisse E. A comparative atomic force microscopy study on living skin fibroblasts and liver endothelial cells. J Electron Microsc (Tokyo). 2001;50:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999;6:23-44. [PubMed] |
| 22. | Yokomori H, Oda M, Yoshimura K, Nagai T, Ogi M, Nomura M, Ishii H. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Oda M, Yokomori H, Han J Y, Kamegaya Y, Ogi M, Nakamura M. Hepatic sinusoidal endothelial fenestrae are a stationary type of fused and interconnected caveolae. Cells of the Hepatic Sinusoid 8. Leiden: Kupffer Cell Foundation 2001; 94-98. |
| 24. | Nagamori S, Hasumura S, Matsuura T, Aizaki H, Kawada M. Developments in bioartificial liver research: concepts, performance, and applications. J Gastroenterol. 2000;35:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Iwahori T, Matsuura T, Maehashi H, Sugo K, Saito M, Hosokawa M, Chiba K, Masaki T, Aizaki H, Ohkawa K. CYP3A4 inducible model for in vitro analysis of human drug metabolism using a bioartificial liver. Hepatology. 2003;37:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Li MX, Nakajima T, Fukushige T, Kobayashi K, Seiler N, Saheki T. Aberrations of ammonia metabolism in ornithine carbamoyltransferase-deficient spf-ash mice and their prevention by treatment with urea cycle intermediate amino acids and an ornithine aminotransferase inactivator. Biochim Biophys Acta. 1999;1455:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Glicklis R, Merchuk JC, Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol Bioeng. 2004;86:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Ma M, Xu J, Purcell WM. Biochemical and functional changes of rat liver spheroids during spheroid formation and maintenance in culture: I. morphological maturation and kinetic changes of energy metabolism, albumin synthesis, and activities of some enzymes. J Cell Biochem. 2003;90:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | MacSween R. N. M. Pathology of the Liver, 4th ed. In: Developmental anatomy and normal structure, Churchill Livingstone 2002; 16-22. |
| 30. | Matsumoto T, Komori R, Magara T, Ui T, Kawakami M, Hano H. A study on the normal structure of the human liver, with special reference to its angioarchitecture. Jikei Med J. 1979;26:1-40. |
| 31. | Ishida Y, Smith S, Wallace L, Sadamoto T, Okamoto M, Auth M, Strazzabosco M, Fabris L, Medina J, Prieto J. Ductular morphogenesis and functional polarization of normal human biliary epithelial cells in three-dimensional culture. J Hepatol. 2001;35:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF