Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1603
Revised: August 1, 2005
Accepted: August 26, 2005
Published online: March 14, 2006
AIM: To investigate the role of mangafodipir trisodium (MnDPDP) in focal pancreatic masses and mass-like lesions by evaluating contrast uptake features of the lesions and pancreatic parenchyma after contrast medium injection.
METHODS: A total of 37 patients with pancreatic mass or mass-like lesions were examined by unenhanced and MnDPDP-enhanced magnetic resonance imaging (MRI).
RESULTS: MRI was obtained 20-40 min after infusion of MnDPDP and homogeneous contrast enhancement was observed in normal pancreas parenchyma. In patients with atrophic pancreas there was no enhancement in pancreatic parenchyma on MnDPDP-enhanced MRI. In 37 patients with 41 pancreatic masses and mass-like lesions, contrast enhancement was observed at 5 lesions on MnDPDP enhanced MRI. Three of these 5 lesions were focal pancreatitis and the other 2 were adenocarcinoma. No contrast enhancement was determined in 36 pancreatic masses and mass-like lesions in 32 patients.
CONCLUSION: MnDPDP contrast-enhanced MRI, especially in cases with no parenchyma atrophy, can distinguish focal pancreatic lesion margins. Information about the function of pancreatic parenchyma can be obtained out of tumor. MnDPDP facilitates staging of pancreatic tumors by detection of metastatic lesions in the liver. In addition, diminished hetergenous uptake of MnDPDP in patients with pancreatitis may be helpful in differential diagnosis.
- Citation: Eser G, Karabacakoglu A, Karakose S, Eser C, Kayacetin E. Mangafodipir trisodium-enhanced magnetic resonance imaging for evaluation of pancreatic mass and mass-like lesions. World J Gastroenterol 2006; 12(10): 1603-1606
- URL: https://www.wjgnet.com/1007-9327/full/v12/i10/1603.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i10.1603
Diagnosis of focal pancreatic lesions is a significant challenge for radiologists and surgeons. Focal pancreatic lesions originate from the exocrine (mainly ductal adenocarcinomas) or endocrine part of gland (apudomas), cysts and/or inflammatory lesions. The diagnosis of apudoma is based on the elevated levels of circulating hormones, while the diagnosis of all other focal pancreatic lesions is mainly based on diagnostic imaging[1].
The relation of tumor with its surrounding structures can be described by cross-sectional techniques such as ultrasonography (US), CT and MRI. Ultrasonography has been proved as a first screening mode to depict the panceatic head and duct, the common bile duct and major vessels, but its value is limited by air in the digestive tract and its operator. Contrast enhanced triphasic spiral CT has an accuracy of only 70% for local staging[2]. Surgical procedures are therefore sometimes needed for diagnostic purposes[3,4]. Consequently there is a need for imaging methods with a higher sensitivity and specifity for identifying and staging pancreatic lesions to diminish the number of unnecessary laparotomies.
MnDPDP (Teslascan, Amersham, GE Heralth Care) is an organ-specific paramagnetic contrast agent that has been developed for imaging of the hepatobiliary system. Significant tissue uptake is seen mainly in the liver but much less in the renal cortex, adrenal glands, pancreas and other abdominal organs[5-7]. MnDPDP enables to get images with a higher signal-to-noise (S/N) ratio[8].
In this study, pancreatic mass and mass-like lesions were examined by MRI performed using MnDPDP. The aim of this study was to investigate the role of MnDPDP in focal pancreatic masses and mass-like lesions by evaluating features of their contrast uptake after contrast medium injection.
Pancreatic mass lesions or suspicious mass were determined in 37 patients (24 men, 13 women) with a mean age of 58.1 years (range 23-83 years) by US and CT. All patients underwent MnDPDP-enhanced and unenhanced MRI. The patients who received any contrast agent within 1 h before MnDPDP-enhanced MRI or had obstructive hepatobiliary disease or biliary stasis or severe renal impairment, or were contraindicative for CT or MRI or previously enrolled into this study, or defined as pregnancy or lactation, were excluded. The study was approved by the local ethical committee.
MR imaging was performed at 1.5 T (Picker Edge, Picker Internationals Highlands Height, OH) using a body coil. Sequences of axial T2-weighted fast-spin-echo (TR/TE: 8600-9700/96 msec matrix 192 × 256, slice thickness 3,5 mm, flip angle 90, FOV 40-55 cm), axial and coronal T1-weighted gradient echo (TR/TE: 155/8.1 msec, matrix 160x256, slice thickness 3 mm, gap 0,5 mm, flip angle 60, FOV 40-55 cm, breath-hold 15-20 sec) were applied in all patients before administration of contrast agent. Images of the coronal plane were obtained using only one of the three sequences (SE, gradient echo or fat sat gradient echo).
MnDPDP was injected intravenously at a dosage of 10 μmol/kg (2-3 mL/min) for 15 minutes. Postcontrast imaging was obtained using the same protocol and sequences (except for T2 weighted fast SE sequence) approximately 30 minutes after contrast administration. After contrast medium injection, the size, number, appearance, characteristics of the lesions were compared with normal pancreatic parenchyma. Also contrast uptake level in the lesions and pancreatic parenchyma were evaluated.
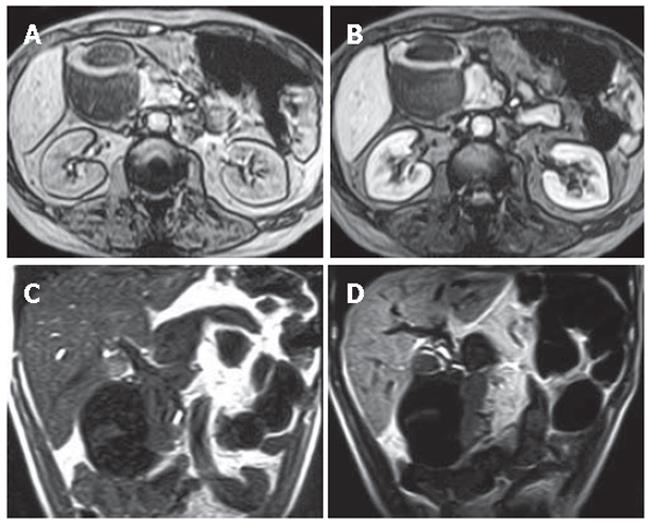
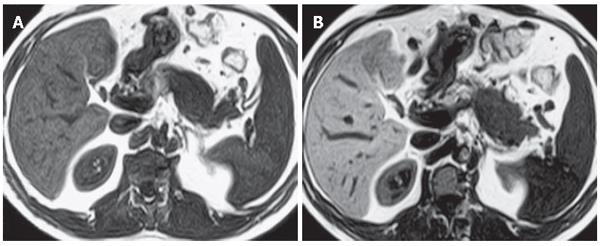
Diagnosis of all patients was confirmed by radiological, clinical and histopathological examination (Table 1). Twenty-three of the 37 patients underwent surgical intervention and percutaneous biopsy was performed in 5 patients with primary or metastatic lesions. In the remaining 9 patients, diagnosis was confirmed by clinical and radiological follow-up. Twenty-four patients with pancreatic carcinoma underwent MnDPDP-enhanced abdominal MRI. In 22 of 24 patients, contrast medium uptake in the mass lesions was not determined (Figure 1). Minimal heterogeneous contrast enhancement was seen in one of the 2 patients with contrast-enhanced masses and contrast enhancement of the septa was observed in the other patient with mucinous cystadenocarcinoma (Figure 2).
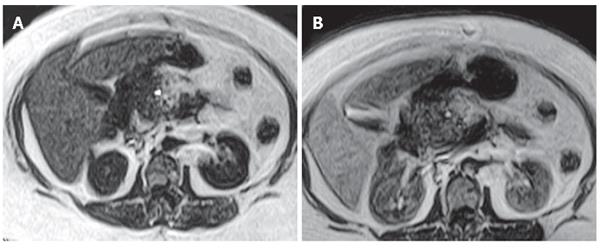
In patients with pancrea carcinoma contrast enhancement of pancreatic parencyhma out of the mass was evaluatedtic. Contrast enhancement was determined in 13 cases and no contrast enhancement was observed in 11 cases. Homogeneous contrast enhancement of pancreatic parencyhma was seen in 11 cases. MnDPDP-enhanced images revealed a significant improvement in the demarcation and depiction of pancreatic masses as compared to non-enhanced images. Contrast uptake of parenchyma out of mass was minimal in the other 2 cases. In these cases of pancreatic head carcinoma, pancreatitis was found with enlarged and heterogeneous contrast enhancement at the pancreatic parencyhma. In 11 patients with pancreas carcinoma, pancreatic parencyhma was atrophic, indicating that these patients after MnDPDP infusion had no contrast enhancement in parencyhma out of the mass. In 3 patients with focal pancreatitis, the lesions were located at the tail in 1 case and at the head in 2 cases. Heterogeneous and reduced contrast enhancement in the focal lesions and homogeneous enhancement of parencyhma out of the lesion were observed in all the 3 cases after MnDPDP infusion (Figure 3). In patients with pancreas pseudocyst, metastatic lesion, hydatid cyst, aggressive type non-Hodgkin’s lymphoma, lipoma, choledochal cyst and gastrinoma (Figure 4), no contrast enhancement was seen in all masses after MnDPDP infusion. In 37 patients with pancreatic masses or mass-like lesions, minimal contrast enhancement was established in 5 of 41 lesions (12.2%) by MnDPDP-enhanced MRI. No contrast enhancement was determined in 36 (87.8%) pancreatic masses or mass-like lesions in 32 patients.
Ductal adenocarcinoma of the pancreas comprises 75-90 % of all tumors of the pancreas[9]. Abdominal discomfort, back pain, obstructive jaundice and weight loss are the most common symptoms of pancreatic cancer[10]. The only potential cure for pancreatic neoplasms is surgery. Therefore early diagnognosis and assessment of tumor resectability are fundamental to achieve a successful treatment[11]. Bluemke et al[3] reported that ductal adenocarcinoma can be detected with helical CT and that further efforts to improve the preoperative staging of pancreatic ductal adenocarcinoma with helical CT should be directed toward improving the detection of small pancreatic ductal adenocarcinomas. Vellet et al[12] also reported that non-contour deforming small adenocarcinomas may not be detected by dynamic CT. Small pancreatic masses can be readily detected by MnDPDP-enhanced MRI and the sensitivity of MRI in staging of tumor is increased[13]. Gehl et al[14] reported that pancreatic enhancement is increased by 98% after MnDPDP injection. It was also found that after MnDPDP infusion, signal intensity of normal pancreatic parencyhma rises while tumor remains with less signal intensity[15].
Tumor-forming pancreatitis, also known as pseudotumorous pancreatitis or inflammatory pancreatic mass, is presented as a mass-like glandular enlargement. Focal enlargement of the pancreas typically involving head of the pancreas and common bile duct may occur, simulating the development of malignant neoplasms. Since the signal intensity and nonspecific contrast enhancement patterns of this tumor are similar to those of malignant tumor, their differential diagnosis is usually diffucult[16]. In our study, minimal heterogeneous contrast enhancement was observed in 3 patients with local pancreatitis by MnDPDP-enhanced MRI.
In patients with pancreatic adenocarcinomas, the contrast enhancement was compared to the proximal and distal part of tumor after MnDPDP infusion and less enhancement was found in the distal part of tumor. This may be caused by the obstruction of the pancreatic duct which is often found adjacent to the distal part of tumor[1]. In our study, pancreatic contrast enhancement at the distal part of tumor was poor in 2 cases because of pancreatitis adjacent to the distal part of tumor. Duct dilatation and atrophy of the pancreatic tail and body can be seen due to obstruction of the pancreatic duct in malignant tumor at the head of the pancreas. The parenchyma may have a poor function even though atrophy does not occur, contrast enhancement of parenchyma becomes markedly less[8].
In our study, no uptake of contrast medium was observed in 11 patients with pancreatic malignant tumor by MnDPDP-enhanced MRI. Contrast enhancement of tumor was not observed in patients with tumor (out of 2 cases). On MnDPDP-enhanced T1-weighted images, minimal contrast-enhanced mass was detected in 1 patient with adenocarcinoma at the head of pancreas and contrast enhancement was observed in septa of mass of the other patient with mucinous cystadenocarcinoma, demonstrating that MnDPDP-enhanced MRI gives a better delineation of the tumor, especially in patients with no parenchyma atrophy. It may be difficult to distinguish the lesions simulating the occurrence of malignant neoplasms in patients with focal pancreatitis from malignant tumors. Diminished heteregenous uptake of MnDPDP of the lesions in patients with pancreatitis may be helpful in differential diagnosis.
MnDPDP is used as an organ specific contrast agent orginally designed for liver imaging. Since the agent is taken up by hepatocytes, increased contrast between normal liver parenchyma and metastatic liver lesions can be achieved. MnDPDP facilitates staging of pancreatic tumors by providing easily detection of metastatic lesions to the liver. MnDPDP may improve the staging of pancreatic cancer by increasing the sensitivity of MRI in the detection of liver metastases. MnDPDP-enhanced MRI can improve the accuracy of detection ratio and staging of focal pancreatic lesions, and is a safe and well tolerated noninvasive diagnostic method.
| 1. | Romijn MG, Stoker J, van Eijck CH, van Muiswinkel JM, Torres CG, Lameris JS. MRI with mangafodipir trisodium in the detection and staging of pancreatic cancer. J Magn Reson Imaging. 2000;12:261-268. [DOI] [Full Text] |
| 2. | Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745-751. [PubMed] |
| 3. | Bluemke DA, Fishman EK, Kuhlman J. CT evaluation following Whipple procedure: potential pitfalls in interpretation. J Comput Assist Tomogr. 1992;16:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Coley SC, Strickland NH, Walker JD, Williamson RC. Spiral CT and the pre-operative assessment of pancreatic adenocarcinoma. Clin Radiol. 1997;52:24-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Bartolozzi C, Lencioni R, Donati F, Cioni D. Abdominal MR: liver and pancreas. Eur Radiol. 1999;9:1496-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Wang C, Johansson L, Western A, Fagertun H, Ahlstrom H. Sequence optimization in mangafodipir trisodium-enhanced liver and pancreas MRI. J Magn Reson Imaging. 1999;9:280-284. [DOI] [Full Text] |
| 7. | Hustvedt SO, Grant D, Southon TE, Zech K. Plasma pharmacokinetics, tissue distribution and excretion of MnDPDP in the rat and dog after intravenous administration. Acta Radiol. 1997;38:690-699. [PubMed] |
| 8. | Ahlström H, Gehl HB. Overview of MnDPDP as a pancreas-specific contrast agent for MR imaging. Acta Radiol. 1997;38:660-664. [PubMed] |
| 9. | Klöppel G, Maillet B. Classification and staging of pancreatic nonendocrine tumors. Radiol Clin North Am. 1989;27:105-119. [PubMed] |
| 10. | Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer. 1985;56:397-402. [DOI] [Full Text] |
| 11. | Warshaw AL, Gu ZY, Wittenberg J, Waltman AC. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 282] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Vellet AD, Romano W, Bach DB, Passi RB, Taves DH, Munk PL. Adenocarcinoma of the pancreatic ducts: comparative evaluation with CT and MR imaging at 1.5 T. Radiology. 1992;183:87-95. [PubMed] |
| 13. | Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol. 2002;12:2998-3008. [PubMed] |
| 14. | Gehl HB, Vorwerk D, Klose KC, Günther RW. Pancreatic enhancement after low-dose infusion of Mn-DPDP. Radiology. 1991;180:337-339. [PubMed] |
| 15. | Gehl HB, Urhahn R, Bohndorf K, Klever P, Hauptmann S, Lodemann KP, Matern S, Schumpelick V, Günther RW. Mn-DPDP in MR imaging of pancreatic adenocarcinoma: initial clinical experience. Radiology. 1993;186:795-798. [PubMed] |
| 16. | Ito K, Koike S, Matsunaga N. MR imaging of pancreatic diseases. Eur J Radiol. 2001;38:78-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Co-first-authors: Gul Eser
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF